DhbB
- Description: isochorismatase
| Gene name | dhbB |
| Synonyms | |
| Essential | no |
| Product | isochorismatase |
| Function | biosynthesis of the siderophore bacillibactin |
| Gene expression levels in SubtiExpress: dhbB | |
| MW, pI | 34 kDa, 4.429 |
| Gene length, protein length | 936 bp, 312 aa |
| Immediate neighbours | dhbF, dhbE |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 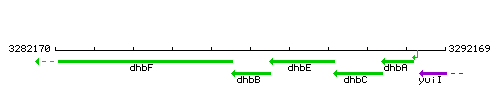
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed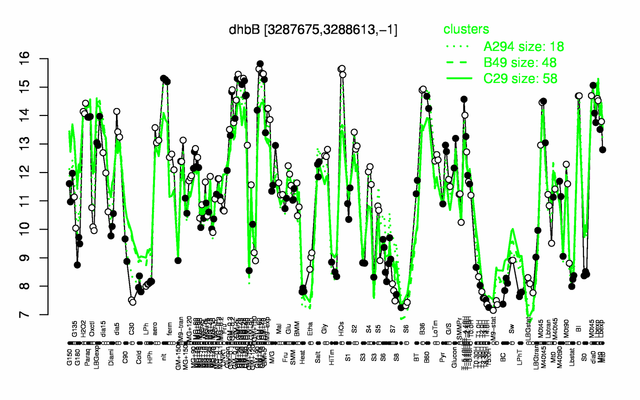
| |
Contents
Categories containing this gene/protein
acquisition of iron, iron metabolism
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31970
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Isochorismate + H2O = 2,3-dihydroxy-2,3-dihydrobenzoate + pyruvate (according to Swiss-Prot)
- Protein family: isochorismatase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P45743
- KEGG entry: [3]
- E.C. number: 3.3.2.1
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Imke G de Jong, Jan-Willem Veening, Oscar P Kuipers
Single cell analysis of gene expression patterns during carbon starvation in Bacillus subtilis reveals large phenotypic variation.
Environ Microbiol: 2012, 14(12);3110-21
[PubMed:23033921]
[WorldCat.org]
[DOI]
(I p)
Martin Lehnik-Habrink, Marc Schaffer, Ulrike Mäder, Christine Diethmaier, Christina Herzberg, Jörg Stülke
RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y.
Mol Microbiol: 2011, 81(6);1459-73
[PubMed:21815947]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Noel Baichoo, Tao Wang, Rick Ye, John D Helmann
Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon.
Mol Microbiol: 2002, 45(6);1613-29
[PubMed:12354229]
[WorldCat.org]
[DOI]
(P p)
Tamara Hoffmann, Alexandra Schütz, Margot Brosius, Andrea Völker, Uwe Völker, Erhard Bremer
High-salinity-induced iron limitation in Bacillus subtilis.
J Bacteriol: 2002, 184(3);718-27
[PubMed:11790741]
[WorldCat.org]
[DOI]
(P p)
J J May, T M Wendrich, M A Marahiel
The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin.
J Biol Chem: 2001, 276(10);7209-17
[PubMed:11112781]
[WorldCat.org]
[DOI]
(P p)
B M Rowland, T H Grossman, M S Osburne, H W Taber
Sequence and genetic organization of a Bacillus subtilis operon encoding 2,3-dihydroxybenzoate biosynthetic enzymes.
Gene: 1996, 178(1-2);119-23
[PubMed:8921902]
[WorldCat.org]
[DOI]
(P p)
B M Rowland, H W Taber
Duplicate isochorismate synthase genes of Bacillus subtilis: regulation and involvement in the biosyntheses of menaquinone and 2,3-dihydroxybenzoate.
J Bacteriol: 1996, 178(3);854-61
[PubMed:8550523]
[WorldCat.org]
[DOI]
(P p)