Difference between revisions of "TkmA"
| Line 45: | Line 45: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| − | {{SubtiWiki regulon|[[AbrB regulon]]}} | + | {{SubtiWiki regulon|[[AbrB regulon]]}}, |
| + | {{SubtiWiki regulon|[[DegU regulon]]}}, | ||
| + | {{SubtiWiki regulon|[[Spo0A regulon]]}} | ||
| + | |||
=The gene= | =The gene= | ||
| Line 116: | Line 119: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | |||
** [[AbrB]]: transcription repression {{PubMed|20817675}} | ** [[AbrB]]: transcription repression {{PubMed|20817675}} | ||
| + | ** [[Spo0A]]: transcription activation {{PubMed|26283769}} | ||
| + | ** [[DegU]]-P: transcription activation {{PubMed|26283769}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 17:04, 19 August 2015
- Description: transmembrane modulator of PtkA activity, activates PtkA autophosphorylation and substrate phosphorylation
| Gene name | tkmA |
| Synonyms | ywqC |
| Essential | no |
| Product | modulator of PtkA activity |
| Function | control of protein tyrosine phosphorylation |
| Gene expression levels in SubtiExpress: tkmA | |
| Interactions involving this protein in SubtInteract: TkmA | |
| MW, pI | 26 kDa, 4.61 |
| Gene length, protein length | 744 bp, 248 aa |
| Immediate neighbours | ptkA, ywzD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed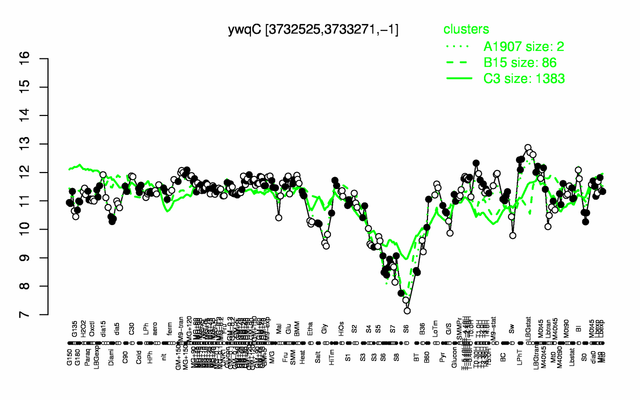
| |
Contents
Categories containing this gene/protein
biofilm formation, protein modification, membrane proteins
This gene is a member of the following regulons
AbrB regulon, DegU regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU36260
Phenotypes of a mutant
- the mutant exhibits a defect in biofilm formation, pronounced on LBGM medium, weak of MSgg medium PubMed
Database entries
- BsubCyc: BSU36260
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transmembrane activation of PtkA protein tyrosine kinase activity
- Protein family: cpsC/capA family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU36260
- Structure:
- UniProt: P96715
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- GP1566 (spc) PubMed, available in Jörg Stülke's lab
- GP1567 epsA::aphA3 tkmA::spc PubMed, available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct: GP1620 tkmA-FLAG 3x spc (based on pGP1331) available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References