Difference between revisions of "EpsB"
(→Original publications) |
|||
| Line 155: | Line 155: | ||
<pubmed>20735481 </pubmed> | <pubmed>20735481 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>15661000,16430695,18047568,18647168 18547145 20817675 21856853 21815947 23646920 24493247 </pubmed> | + | <pubmed>15661000,16430695,18047568,18647168 18547145 20817675 21856853 21815947 23646920 24493247 24728941 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 07:12, 15 April 2014
- Description: extracellular polysaccharide synthesis, putative protein tyrosine kinase
| Gene name | epsB |
| Synonyms | yveL |
| Essential | no |
| Product | unknown |
| Function | biofilm formation |
| Gene expression levels in SubtiExpress: epsB | |
| Interactions involving this protein in SubtInteract: EpsB | |
| Regulation of this protein in SubtiPathways: epsB | |
| MW, pI | 24 kDa, 9.918 |
| Gene length, protein length | 681 bp, 227 aa |
| Immediate neighbours | epsC, epsA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 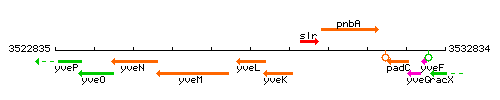
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
protein modification, biofilm formation
This gene is a member of the following regulons
AbrB regulon, RemA regulon, SinR regulon
The gene
Basic information
- Locus tag: BSU34360
Phenotypes of a mutant
Database entries
- BsubCyc: BSU34360
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + a [protein]-L-tyrosine = ADP + a [protein]-L-tyrosine phosphate (according to Swiss-Prot)
- Protein family: BY kinase, see the Bacterial Protein Tyrosine Kinase Database)
- Paralogous protein(s): PtkA
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU34360
- UniProt: P71051
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
- induction by sequestration of SinR by SinI or SlrA PubMed
- the epsA-epsB-epsC-epsD-epsE-epsF-epsG-epsH-epsI-epsJ-epsK-epsL-epsM-epsN-epsO operon is not expressed in a ymdB mutant PubMed
- the amount of the mRNA is substantially decreased upon depletion of RNase Y (this is likely due to the increased stability of the sinR mRNA) PubMed
Biological materials
- Mutant:
- GP1518 (aphA3) PubMed, available in Jörg Stülke's lab
- GP1519 (epsA-epsB, aphA3) PubMed, available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH) PubMed, available in Jörg Stülke's lab
- Antibody:
- FLAG-tag construct: GP1541 epsB-FLAG 3x spc (based on pGP1331) available in Jörg Stülke's lab
Labs working on this gene/protein
Richard Losick, Harvard Univ., Cambridge, USA homepage
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications