Difference between revisions of "DnaE"
(→Original publications) |
|||
| Line 108: | Line 108: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' |
| + | ** [http://www.pdb.org/pdb/explore/explore.do?structureId=3e0d 3E0D] (from ''Thermus aquaticus'', 39% identity) {{PubMed|18691598}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/O34623 O34623] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O34623 O34623] | ||
| Line 156: | Line 157: | ||
<pubmed> 22933559 </pubmed> | <pubmed> 22933559 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>,11721055,14593098,16267290, 20122408 23563155 23017159,21958350,23268446 24062730 24106089</pubmed> | + | <pubmed>,11721055,14593098,16267290, 20122408 23563155 23017159,21958350,23268446 24062730 24106089 18691598</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 19:16, 17 November 2013
- Description: DNA polymerase III (alpha subunit), part of the replisome
| Gene name | dnaE |
| Synonyms | |
| Essential | yes PubMed |
| Product | DNA polymerase III (alpha subunit) |
| Function | DNA replication |
| Gene expression levels in SubtiExpress: dnaE | |
| Interactions involving this protein in SubtInteract: DnaE | |
| MW, pI | 125 kDa, 6.02 |
| Gene length, protein length | 3345 bp, 1115 aa |
| Immediate neighbours | ytsJ, ytrH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 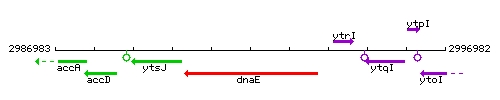
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed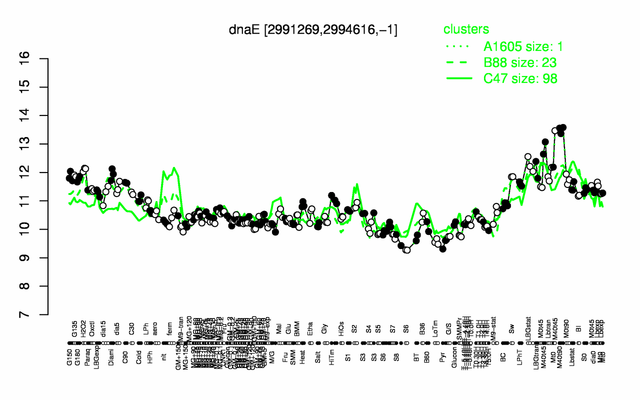
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29230
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot)
- required for bacteriophage SPP1 replication PubMed
- Protein family: DnaE subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O34623
- KEGG entry: [3]
- E.C. number: 2.7.7.7
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications