Difference between revisions of "DhbA"
| Line 37: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 115: | Line 111: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dhbA_3291511_3292296_-1 dhbA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dhbA_3291511_3292296_-1 dhbA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] {{PubMed|8550523}} | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|8550523}} |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 147: | Line 143: | ||
=References= | =References= | ||
| − | + | <pubmed>8224884,10400588,11112781 ,11790741,12354229, 8921902 8550523 20525796 20817675 21815947</pubmed> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | <pubmed>8224884,10400588,11112781 ,11790741,12354229, 8921902 8550523 20525796 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 20:50, 18 June 2013
- Description: 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase
| Gene name | dhbA |
| Synonyms | entA |
| Essential | no |
| Product | 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase |
| Function | biosynthesis of the siderophore bacillibactin |
| Gene expression levels in SubtiExpress: dhbA | |
| MW, pI | 27 kDa, 5.311 |
| Gene length, protein length | 783 bp, 261 aa |
| Immediate neighbours | dhbC, besA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 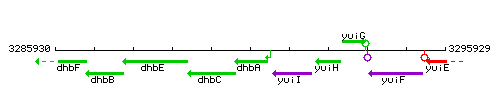
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
acquisition of iron, iron metabolism
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU32000
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2,3-dihydro-2,3-dihydroxybenzoate + NAD+ = 2,3-dihydroxybenzoate + NADH (according to Swiss-Prot)
- Protein family: short-chain dehydrogenases/reductases (SDR) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P39071
- KEGG entry: [3]
- E.C. number: 1.3.1.28
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Martin Lehnik-Habrink, Marc Schaffer, Ulrike Mäder, Christine Diethmaier, Christina Herzberg, Jörg Stülke
RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y.
Mol Microbiol: 2011, 81(6);1459-73
[PubMed:21815947]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Noel Baichoo, Tao Wang, Rick Ye, John D Helmann
Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon.
Mol Microbiol: 2002, 45(6);1613-29
[PubMed:12354229]
[WorldCat.org]
[DOI]
(P p)
Tamara Hoffmann, Alexandra Schütz, Margot Brosius, Andrea Völker, Uwe Völker, Erhard Bremer
High-salinity-induced iron limitation in Bacillus subtilis.
J Bacteriol: 2002, 184(3);718-27
[PubMed:11790741]
[WorldCat.org]
[DOI]
(P p)
J J May, T M Wendrich, M A Marahiel
The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin.
J Biol Chem: 2001, 276(10);7209-17
[PubMed:11112781]
[WorldCat.org]
[DOI]
(P p)
N Bsat, J D Helmann
Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo.
J Bacteriol: 1999, 181(14);4299-307
[PubMed:10400588]
[WorldCat.org]
[DOI]
(P p)
B M Rowland, T H Grossman, M S Osburne, H W Taber
Sequence and genetic organization of a Bacillus subtilis operon encoding 2,3-dihydroxybenzoate biosynthetic enzymes.
Gene: 1996, 178(1-2);119-23
[PubMed:8921902]
[WorldCat.org]
[DOI]
(P p)
B M Rowland, H W Taber
Duplicate isochorismate synthase genes of Bacillus subtilis: regulation and involvement in the biosyntheses of menaquinone and 2,3-dihydroxybenzoate.
J Bacteriol: 1996, 178(3);854-61
[PubMed:8550523]
[WorldCat.org]
[DOI]
(P p)
R Adams, W Schumann
Cloning and mapping of the Bacillus subtilis locus homologous to Escherichia coli ent genes.
Gene: 1993, 133(1);119-21
[PubMed:8224884]
[WorldCat.org]
[DOI]
(P p)