Difference between revisions of "AccD"
| Line 113: | Line 113: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' ''[[accD]]-[[accA]]'' | + | * '''Operon:''' ''[[accD]]-[[accA]]'' {{PubMed|22383849}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=accD_2988693_2989565_-1 accD] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=accD_2988693_2989565_-1 accD] {{PubMed|22383849}} | ||
| Line 149: | Line 149: | ||
<pubmed> 15952903 </pubmed> | <pubmed> 15952903 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>15066985, 12663926,14651647,16479537 22517742</pubmed> | + | <pubmed>15066985, 12663926,14651647,16479537 22517742 22383849</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:08, 21 April 2012
- Description: acetyl-CoA carboxylase (beta subunit)
| Gene name | accD |
| Synonyms | yttI |
| Essential | yes PubMed |
| Product | acetyl-CoA carboxylase (beta subunit)) |
| Function | production of malonyl-CoA, the substrate for fatty acid biosynthesis |
| Interactions involving this protein in SubtInteract: AccD | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 28 kDa, 5.344 |
| Gene length, protein length | 786 bp, 262 aa |
| Immediate neighbours | accA, ytsJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 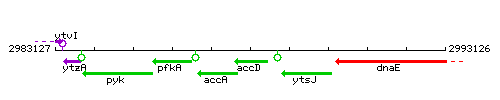
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed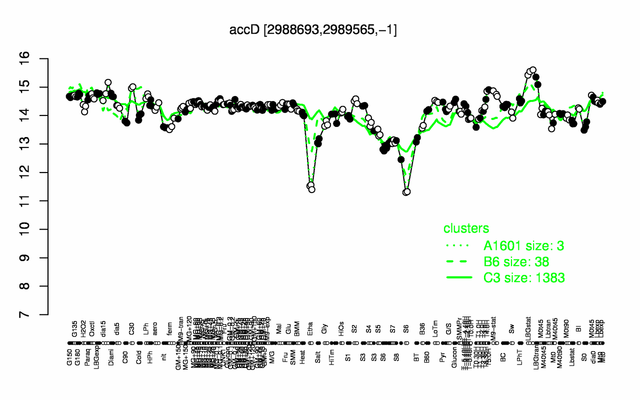
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29210
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-205 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization: Membrane-proximal (Spotty) PubMed Membrane-proximal (Spotty) PubMed
Database entries
- UniProt: C0SP93
- KEGG entry: [3]
- E.C. number: 6.4.1.2
Additional information
Expression and regulation
- Sigma factor:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications