TasA
Revision as of 10:10, 17 April 2014 by 134.76.70.252 (talk)
- Description: major component of biofilm matrix, forms amyloid fibers
| Gene name | tasA |
| Synonyms | cotN, yqhF |
| Essential | no |
| Product | major component of biofilm matrix |
| Function | biofilm formation |
| Gene expression levels in SubtiExpress: tasA | |
| Interactions involving this protein in SubtInteract: TasA | |
| Regulation of this protein in SubtiPathways: tasA | |
| MW, pI | 28 kDa, 5.442 |
| Gene length, protein length | 783 bp, 261 aa |
| Immediate neighbours | sinR, sipW |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 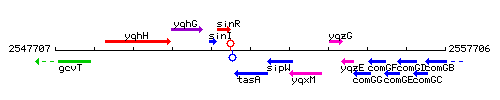
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed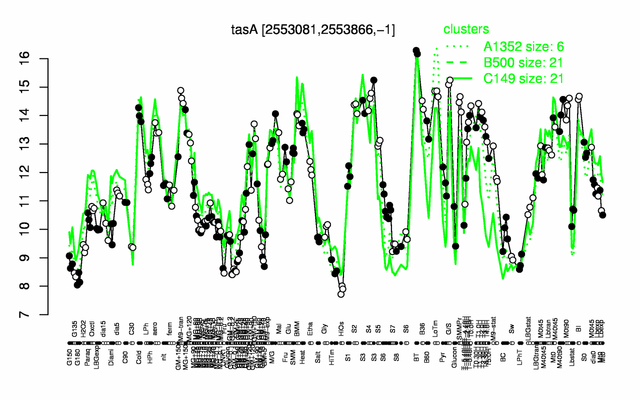
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
AbrB regulon, RemA regulon, SinR regulon
The gene
Basic information
- Locus tag: BSU24620
Phenotypes of a mutant
- altered cell death pattern in colonies PubMed
Database entries
- BsubCyc: BSU24620
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: forms amyloid fibers that bind cells together in the biofilm PubMed
- Protein family: peptidase M73 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU24620
- Structure:
- UniProt: P54507
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- induction by sequestration of SinR by SinI or SlrA PubMed or by SlrR PubMed
- the tapA-sipW-tasA operon is not expressed in a ymdB mutant PubMed
- the amount of the mRNA is substantially decreased upon depletion of RNase Y (this is likely due to the increased stability of the sinR mRNA) PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 199 PubMed
Biological materials
- Mutant:
- 1S121 (tasA::spec), PubMed, available at BGSC
- GP1571 (cat), available in Jörg Stülke's lab
- GP1672 (deletion of sinR-tasA::cat) PubMed, available in Jörg Stülke's lab
- GP1663 (deletion of yqhG-sinI-sinR-tasA), available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Adam Driks
Tapping into the biofilm: insights into assembly and disassembly of a novel amyloid fibre in Bacillus subtilis.
Mol Microbiol: 2011, 80(5);1133-6
[PubMed:21488983]
[WorldCat.org]
[DOI]
(I p)
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Yunrong Chai, Thomas Norman, Roberto Kolter, Richard Losick
An epigenetic switch governing daughter cell separation in Bacillus subtilis.
Genes Dev: 2010, 24(8);754-65
[PubMed:20351052]
[WorldCat.org]
[DOI]
(I p)
Original publications