CheA
- Description: two-component sensor kinase, chemotactic signal modulator
| Gene name | cheA |
| Synonyms | cheN |
| Essential | no |
| Product | two-component sensor kinase |
| Function | chemotactic signal modulator |
| Gene expression levels in SubtiExpress: cheA | |
| Interactions involving this protein in SubtInteract: CheA | |
| MW, pI | 74 kDa, 4.452 |
| Gene length, protein length | 2013 bp, 671 aa |
| Immediate neighbours | cheB, cheW |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 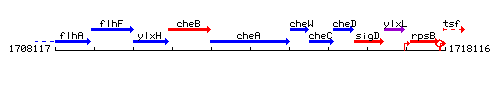
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, motility and chemotaxis, membrane proteins, phosphoproteins
This gene is a member of the following regulons
CodY regulon, DegU regulon, SigD regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU16430
Phenotypes of a mutant
- not essential for pellicle biofilm formation, but mutant is outcompeted by the wild-type strain when competed during pellicle formation PubMed
Database entries
- BsubCyc: BSU16430
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of CheY and CheB PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane (according to Swiss-Prot)
- localizes to the cell poles PubMed
Database entries
- BsubCyc: BSU16430
- Structure:
- UniProt: P29072
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: part of the fla-che operon
- Regulation: see fla-che operon
- Regulatory mechanism: see fla-che operon
- Additional information:
- in minimal medium, CheA is present with 2,600 +/- 560 molecules per cell PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 705 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 1580 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 483 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 188 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 62 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Gerald L Hazelbauer, Wing-Cheung Lai
Bacterial chemoreceptors: providing enhanced features to two-component signaling.
Curr Opin Microbiol: 2010, 13(2);124-32
[PubMed:20122866]
[WorldCat.org]
[DOI]
(I p)
Original publications