PhrE
- Description: response regulator aspartate phosphatase (RapE) regulator, control of the phosphorelay
| Gene name | phrE |
| Synonyms | |
| Essential | no |
| Product | phosphatase (RapE) regulator |
| Function | control of sporulation initiation |
| Interactions involving this protein in SubtInteract: PhrE | |
| Function and regulation of this protein in SubtiPathways: Phosphorelay | |
| MW, pI | 4 kDa, 8.863 |
| Gene length, protein length | 132 bp, 44 aa |
| Immediate neighbours | rapE, yqzI |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 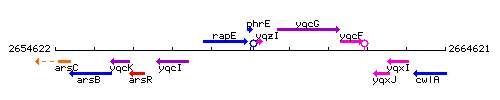
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed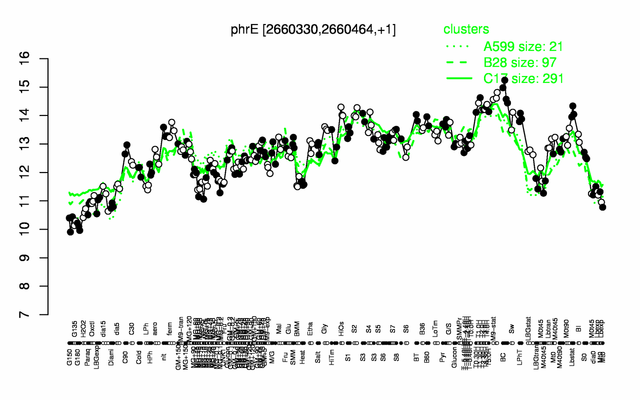
| |
Contents
Categories containing this gene/protein
phosphorelay, quorum sensing, Skin element, short peptides
This gene is a member of the following regulons
AbrB regulon, CcpA regulon, CodY regulon, ComA regulon, SigH regulon
The gene
Basic information
- Locus tag: BSU25840
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: phr family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O32025
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Christopher T Brown, Laura K Fishwick, Binna M Chokshi, Marissa A Cuff, Jay M Jackson, Travis Oglesby, Alison T Rioux, Enrique Rodriguez, Gregory S Stupp, Austin H Trupp, James S Woollcombe-Clarke, Tracy N Wright, William J Zaragoza, Jennifer C Drew, Eric W Triplett, Wayne L Nicholson
Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation.
Appl Environ Microbiol: 2011, 77(19);6867-77
[PubMed:21821766]
[WorldCat.org]
[DOI]
(I p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Yoshiko Nakaura, Robert P Shivers, Hirotake Yamaguchi, Richard Losick, Yasutaro Fujita, Abraham L Sonenshein
Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis.
J Bacteriol: 2003, 185(6);1911-22
[PubMed:12618455]
[WorldCat.org]
[DOI]
(P p)
R S McQuade, N Comella, A D Grossman
Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis.
J Bacteriol: 2001, 183(16);4905-9
[PubMed:11466295]
[WorldCat.org]
[DOI]
(P p)
M Jiang, R Grau, M Perego
Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis.
J Bacteriol: 2000, 182(2);303-10
[PubMed:10629174]
[WorldCat.org]
[DOI]
(P p)