Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' extracellular alkaline serine protease (subtilisin E) <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''aprE'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''sprE '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || extracellular alkaline serine protease (subtilisin E)) |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || protein degradation |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 39 kDa, 9.342 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1143 bp, 381 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[yhfN]]'', ''[[yhfO]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB12870]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |- |
| + | |- | ||
| + | |colspan="2" | '''Genetic context''' <br/> [[Image:aprE_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 35: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU10300 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | |||
| − | |||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/aprE.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10190] |
=== Additional information=== | === Additional information=== | ||
| Line 54: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' Hydrolysis of proteins with broad specificity for peptide bonds, and a preference for a large uncharged residue in P1 (according to Swiss-Prot) |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * ''' | + | * '''Protein family:''' peptidase S8 family (according to Swiss-Prot) |
| − | * ''' | + | * '''Paralogous protein(s):''' |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 76: | Line 70: | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:''' |
| − | * '''Interactions:''' [[ | + | * '''Interactions:''' [[AprE]]-[[PaiA]] |
| − | * '''Localization:''' | + | * '''Localization:''' secreted (according to Swiss-Prot), extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] |
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId= | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=1SBC 1SBC] |
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P04189 P04189] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU10300] |
| + | |||
| + | * '''E.C. number:''' | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' |
| − | * '''[[Sigma factor]]:''' | + | * '''[[Sigma factor]]:''' |
| − | * '''Regulation:''' repressed during logrithmic growth ([[AbrB]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/2504584PubMed | + | * '''Regulation:''' repressed during logrithmic growth ([[AbrB]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/2504584PubMed] |
| − | * '''Regulatory mechanism:''' [[AbrB]]: transcription repression | + | * '''Regulatory mechanism:''' [[AbrB]]: transcription repression |
| − | * '''Additional information:''' | + | * '''Additional information:''' the mRNA is extremely stable (more than 25 min) [http://www.ncbi.nlm.nih.gov/sites/entrez/11101663 PubMed] |
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 120: | Line 115: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | |||
| − | |||
| − | |||
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 129: | Line 120: | ||
=References= | =References= | ||
| − | <pubmed> | + | <pubmed>18957862 11101663 12055299, </pubmed> |
| − | # | + | # Voigt et al. (2009) Cell physiology and protein secretion of ''Bacillus licheniformis'' compared to ''Bacillus subtilis''. ''J Mol Microbiol Biotechnol.'' '''16:''' 53-68 [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] |
| − | + | # Hambraeus, G., Persson, M. & Rutberg, B. (2000). The ''aprE'' leader is a determinant of extreme mRNA stability in ''Bacillus subtilis''. Microbiology 146, 3051-3059. [http://www.ncbi.nlm.nih.gov/sites/entrez/11101663 PubMed] | |
| − | # | + | # Hambraeus, G., Karhumaa, K. & Rutberg, B. (2002). A 5' stem-loop and ribosome binding but not translation are important for the stability of ''Bacillus subtilis aprE'' leader mRNA. Microbiology 148, 1795-1803. [http://www.ncbi.nlm.nih.gov/sites/entrez/12055299 PubMed] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | # | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 19:28, 10 June 2009
- Description: extracellular alkaline serine protease (subtilisin E)
| Gene name | aprE |
| Synonyms | sprE |
| Essential | no |
| Product | extracellular alkaline serine protease (subtilisin E)) |
| Function | protein degradation |
| MW, pI | 39 kDa, 9.342 |
| Gene length, protein length | 1143 bp, 381 aa |
| Immediate neighbours | yhfN, yhfO |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 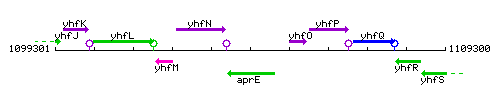
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU10300
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of proteins with broad specificity for peptide bonds, and a preference for a large uncharged residue in P1 (according to Swiss-Prot)
- Protein family: peptidase S8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: secreted (according to Swiss-Prot), extracellular (signal peptide) PubMed
Database entries
- Structure: 1SBC
- Swiss prot entry: P04189
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism: AbrB: transcription repression
- Additional information: the mRNA is extremely stable (more than 25 min) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Voigt et al. (2009) Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis. J Mol Microbiol Biotechnol. 16: 53-68 PubMed
- Hambraeus, G., Persson, M. & Rutberg, B. (2000). The aprE leader is a determinant of extreme mRNA stability in Bacillus subtilis. Microbiology 146, 3051-3059. PubMed
- Hambraeus, G., Karhumaa, K. & Rutberg, B. (2002). A 5' stem-loop and ribosome binding but not translation are important for the stability of Bacillus subtilis aprE leader mRNA. Microbiology 148, 1795-1803. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed