Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' transcriptional regulator of transition state genes <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''abrB'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''cpsX '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || transcriptional regulator |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || regulation of gene expression during the transition from growth to stationary phase |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 10 kDa, 6.57 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 288 bp, 96 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[yabC]]'', ''[[metS]]'' |
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/ | + | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/abrB_nucleotide.txt Gene sequence (+200bp) ]''' |
| − | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/ | + | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/abrB_protein.txt Protein sequence]''' |
|- | |- | ||
| − | + | |colspan="2" | '''Genetic context''' <br/> [[Image:abrB_context.gif]] | |
| − | |||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | ||
|- | |- | ||
|} | |} | ||
| Line 40: | Line 38: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | |||
| + | No swarming motility on B medium. [http://www.ncbi.nlm.nih.gov/sites/entrez/19202088 PubMed] | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/abrB.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10100] |
=== Additional information=== | === Additional information=== | ||
| Line 58: | Line 58: | ||
* '''Protein family:''' | * '''Protein family:''' | ||
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' [[Abh]], [[SpoVT]] (only N-terminal domain) |
| + | |||
| + | === Genes/ operons controlled by AbrB === | ||
| + | |||
| + | * '''Activated by AbrB:''' ''[[citB]]'', ''[[comK]], [[hpr]]'', ''[[rbsR]]-[[rbsK]]-[[rbsD]]-[[rbsA]]-[[rbsC]]-[[rbsB]]'' | ||
| + | |||
| + | * ''' Repressed by AbrB:''' ''[[abrB]], [[aprE]], [[ftsA]]-[[ftsZ]], [[kinC]], [[motA]], [[nprE]], [[pbpE]], [[spo0H]], [[spoVG]], [[spo0E]], [[tycA]], [[sbo]]-[[alb]], [[yqxM]]-[[sipW]]-[[tasA]]'' | ||
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 70: | Line 76: | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:''' interaction with [[AbbA]] results in inactivation of AbrB [http://www.ncbi.nlm.nih.gov/sites/entrez/18840696 PubMed] |
| − | * '''Interactions:''' | + | * '''Interactions:''' [[AbrB]]-[[AbbA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/18840696 PubMed] |
* '''Localization:''' | * '''Localization:''' | ||
| Line 78: | Line 84: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' 1Z0R (N-terminal DNA recognition domain) [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=32611 NCBI] [http://www.ncbi.nlm.nih.gov/sites/entrez/16223496 PubMed] |
* '''Swiss prot entry:''' | * '''Swiss prot entry:''' | ||
| Line 84: | Line 90: | ||
* '''KEGG entry:''' | * '''KEGG entry:''' | ||
| − | + | === Additional information=== | |
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''abrB'' [http://www.ncbi.nlm.nih.gov/sites/entrez/3145384 PubMed] |
| − | * '''Sigma factor:''' | + | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/3145384 PubMed] |
| − | * '''Regulation:''' | + | * '''Regulation:''' expressed at the onset of stationary phase [http://www.ncbi.nlm.nih.gov/sites/entrez/3145384 PubMed] |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' repressed by [[Spo0A]]-P [http://www.ncbi.nlm.nih.gov/sites/entrez/3145384 PubMed] |
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' TT731 (aphA3) |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 115: | Line 120: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| + | |||
| + | [[Richard Losick]], Harvard Univ., Cambridge, USA [http://www.mcb.harvard.edu/Losick/ homepage] | ||
| + | |||
| + | [[Mark Strauch]], Baltimore, USA [http://lifesciences.umaryland.edu/Pages/faculty_profile.aspx?ID=212 homepage] | ||
=Your additional remarks= | =Your additional remarks= | ||
| Line 120: | Line 129: | ||
=References= | =References= | ||
| + | # Banse et al. (2008) Parallel pathways of repression and antirepression governing the transition to stationary phase in ''Bacillus subtilis''.''Proc. Natl. Acad. Sci. USA'' '''105:''' 15547-15552. [http://www.ncbi.nlm.nih.gov/sites/entrez/18840696 PubMed] | ||
| + | # Perego et al. (1988) Structure of the gene for the transition state regulator, ''abrB'': regulator synthesis is controlled by the ''spo0A'' sporulation gene in ''Bacillus subtilis''. ''Mol. Microbiol.'' '''2:''' 689-699. [http://www.ncbi.nlm.nih.gov/sites/entrez/3145384 PubMed] | ||
| + | # Xu, K. and M.A. Strauch. (1996) In vitro selection of optimal AbrB-binding sites: comparison to known in vivo sites indicates flexibility in AbrB-binding and recognition of three-dimensional DNA structures. Molec. Microbiol. 19: 145-158 [http://www.ncbi.nlm.nih.gov/sites/entrez/8821944 PubMed] | ||
| + | # Xu, K., D. Clark and M.A. Strauch. (1996) Analysis of abrB mutations, mutant proteins, and why abrB does not utilize a perfect consensus in the –35 region of its sigmaA promoter.J. Biol. Chem. 271:2621-2626 [http://www.ncbi.nlm.nih.gov/sites/entrez/8576231 PubMed] | ||
| + | # Vaughn, J.L., Feher V., Naylor, S., Strauch, M.A. and J. Cavanagh. (2000) Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator AbrB. Nature Structural Biology 7:1139-1146; [http://www.ncbi.nlm.nih.gov/sites/entrez/11101897 PubMed], Corrigendum appears in Nature Stuctural & Molecular Biology (2005) 12:380 | ||
| + | # Xu, K. and M.A. Strauch. (2001) DNA-binding activity of amino-terminal domains of the Bacillus subtilis AbrB protein. J. Bacteriol. 183:4094-4098 [http://www.ncbi.nlm.nih.gov/sites/entrez/11395475 PubMed] | ||
| + | # Phillips, Z. E.V. and M.A. Strauch. (2001) Role of Cys54 in AbrB multimerization and DNA-binding activity. FEMS Microbiol. Letters. 203:207-210 [http://www.ncbi.nlm.nih.gov/sites/entrez/11583849 PubMed] | ||
| + | # Phillips, Z.E.V. and M.A. Strauch. (2002) Bacillus subtilis sporulation and stationary phase gene expression. Cellular and Molecular Life Sciences 59:392-402 [http://www.ncbi.nlm.nih.gov/sites/entrez/11964117 PubMed] | ||
| + | # Shafikhani, S.H., Mandic-Mulec, I., Strauch, M.A., Smith, I. and T. Leighton. (2002) Postexponential regulation of sin operon expression in Bacillus subtilis. J. Bacteriol. 184:564-571 [http://www.ncbi.nlm.nih.gov/sites/entrez/11751836 PubMed] | ||
| + | # Benson, L. M., Vaughn, J. L., Strauch, M. A., Bobay, B. G., Thompson, R., Naylor, S. and J. Cavanagh (2002). Macromolecular assembly of the transition state regulator AbrB in its unbound and complexed states probed by microelectrospray ionization mass spectrometry. Analytical Biochemistry 306:222-227 [http://www.ncbi.nlm.nih.gov/sites/entrez/12123659 PubMed] | ||
| + | # Qian, Q., Lee, C.Y., Helmann, J. and M.A. Strauch. (2002) AbrB regulation of the sigmaW regulon of Bacillus subtilis. FEMS Microbiol. Letters 211:219-223. [http://www.ncbi.nlm.nih.gov/sites/entrez/12076816 PubMed] | ||
| + | # Kim, H. J., S. I. Kim, M. Ratnayake-Lecamwasam, K. Tachikawa, A. L. Sonenshein, and M. Strauch. (2003) Complex regulation of the Bacillus subtilis aconitase gene. J. Bacteriol. 185:1672-1680. [http://www.ncbi.nlm.nih.gov/sites/entrez/12591885 PubMed] | ||
| + | # Bobay, B.G., Benson, L., Naylor, S., Feeney, B., Clark, A.C., Goshe, M.B., Strauch, M.A., Thompson, R., and J.Cavanagh. (2004) Evaluation of the DNA binding tendencies of the transition state regulator AbrB. Biochemistry. 43:16106-16118. [http://www.ncbi.nlm.nih.gov/sites/entrez/15610005 PubMed] | ||
| + | # Bobay et al.(2005) Revised structure of the AbrB N-terminal domain unifies a diverse superfamily of putative DNA-binding proteins. FEBS Lett. 579:5669-5674. [http://www.ncbi.nlm.nih.gov/sites/entrez/16223496 PubMed] | ||
| + | # Yao, F and M.A. Strauch (2005) Independent and Interchangeable Multimerization Domains of the AbrB, Abh and SpoVT Global Regulatory Proteins. J. Bacteriol. 187:6354-6362 [http://www.ncbi.nlm.nih.gov/sites/entrez/16159768 PubMed] | ||
| + | # Bobay, B.G., Mueller, G.A., Thompson, R.J., Venters, R.A., Murzin, A.G., Strauch, M.A. & J. Cavanagh (2006) NMR structure of AbhN and comparison with AbrBN: First Insights into the DNA-binding Promiscuity and Specificity of AbrB-like Transition-state Regulator Proteins. J. Biol. Chem. 281:21399-21409 [http://www.ncbi.nlm.nih.gov/sites/entrez/16702211 PubMed] | ||
| + | # Jordan S. Rietkötter E. Strauch MA. Kalamorz F. Butcher BG. Helmann JD. Mascher T. (2007) LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis. Microbiology. 153: 2530-2540. [http://www.ncbi.nlm.nih.gov/sites/entrez/17660417 PubMed] | ||
| + | # Strauch MA. Bobay BG. Cavanagh J. Yao F. Wilson A. Le Breton Y. (2007) Abh and AbrB control of Bacillus subtilis antimicrobial gene expression. J. of Bacteriol. 189:7720-7732. [http://www.ncbi.nlm.nih.gov/sites/entrez/17720793 PubMed] | ||
| + | # Hamze et al. (2009) Identification of genes required for different stages of dendritic swarming in ''Bacillus subtilis'', with a novel role for ''phrC''. ''Microbiology'' '''155:''' 398-412. [http://www.ncbi.nlm.nih.gov/sites/entrez/19202088 PubMed] | ||
| + | # Strauch MA. (1995) AbrB modulates expression and catabolite repression of a Bacillus subtilis ribose transport operon. ''J Bacteriol.'' '''Dec;177(23):'''6727-31. [http://www.ncbi.nlm.nih.gov/sites/entrez/7592460 PubMed] | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 17:56, 18 March 2009
- Description: transcriptional regulator of transition state genes
| Gene name | abrB |
| Synonyms | cpsX |
| Essential | no |
| Product | transcriptional regulator |
| Function | regulation of gene expression during the transition from growth to stationary phase |
| MW, pI | 10 kDa, 6.57 |
| Gene length, protein length | 288 bp, 96 aa |
| Immediate neighbours | yabC, metS |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 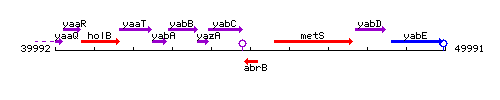
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
No swarming motility on B medium. PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
Genes/ operons controlled by AbrB
- Repressed by AbrB: abrB, aprE, ftsA-ftsZ, kinC, motA, nprE, pbpE, spo0H, spoVG, spo0E, tycA, sbo-alb, yqxM-sipW-tasA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Localization:
Database entries
- Swiss prot entry:
- KEGG entry:
Additional information
Expression and regulation
- Operon: abrB PubMed
- Regulation: expressed at the onset of stationary phase PubMed
- Additional information:
Biological materials
- Mutant: TT731 (aphA3)
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Richard Losick, Harvard Univ., Cambridge, USA homepage
Mark Strauch, Baltimore, USA homepage
Your additional remarks
References
- Banse et al. (2008) Parallel pathways of repression and antirepression governing the transition to stationary phase in Bacillus subtilis.Proc. Natl. Acad. Sci. USA 105: 15547-15552. PubMed
- Perego et al. (1988) Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol. Microbiol. 2: 689-699. PubMed
- Xu, K. and M.A. Strauch. (1996) In vitro selection of optimal AbrB-binding sites: comparison to known in vivo sites indicates flexibility in AbrB-binding and recognition of three-dimensional DNA structures. Molec. Microbiol. 19: 145-158 PubMed
- Xu, K., D. Clark and M.A. Strauch. (1996) Analysis of abrB mutations, mutant proteins, and why abrB does not utilize a perfect consensus in the –35 region of its sigmaA promoter.J. Biol. Chem. 271:2621-2626 PubMed
- Vaughn, J.L., Feher V., Naylor, S., Strauch, M.A. and J. Cavanagh. (2000) Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator AbrB. Nature Structural Biology 7:1139-1146; PubMed, Corrigendum appears in Nature Stuctural & Molecular Biology (2005) 12:380
- Xu, K. and M.A. Strauch. (2001) DNA-binding activity of amino-terminal domains of the Bacillus subtilis AbrB protein. J. Bacteriol. 183:4094-4098 PubMed
- Phillips, Z. E.V. and M.A. Strauch. (2001) Role of Cys54 in AbrB multimerization and DNA-binding activity. FEMS Microbiol. Letters. 203:207-210 PubMed
- Phillips, Z.E.V. and M.A. Strauch. (2002) Bacillus subtilis sporulation and stationary phase gene expression. Cellular and Molecular Life Sciences 59:392-402 PubMed
- Shafikhani, S.H., Mandic-Mulec, I., Strauch, M.A., Smith, I. and T. Leighton. (2002) Postexponential regulation of sin operon expression in Bacillus subtilis. J. Bacteriol. 184:564-571 PubMed
- Benson, L. M., Vaughn, J. L., Strauch, M. A., Bobay, B. G., Thompson, R., Naylor, S. and J. Cavanagh (2002). Macromolecular assembly of the transition state regulator AbrB in its unbound and complexed states probed by microelectrospray ionization mass spectrometry. Analytical Biochemistry 306:222-227 PubMed
- Qian, Q., Lee, C.Y., Helmann, J. and M.A. Strauch. (2002) AbrB regulation of the sigmaW regulon of Bacillus subtilis. FEMS Microbiol. Letters 211:219-223. PubMed
- Kim, H. J., S. I. Kim, M. Ratnayake-Lecamwasam, K. Tachikawa, A. L. Sonenshein, and M. Strauch. (2003) Complex regulation of the Bacillus subtilis aconitase gene. J. Bacteriol. 185:1672-1680. PubMed
- Bobay, B.G., Benson, L., Naylor, S., Feeney, B., Clark, A.C., Goshe, M.B., Strauch, M.A., Thompson, R., and J.Cavanagh. (2004) Evaluation of the DNA binding tendencies of the transition state regulator AbrB. Biochemistry. 43:16106-16118. PubMed
- Bobay et al.(2005) Revised structure of the AbrB N-terminal domain unifies a diverse superfamily of putative DNA-binding proteins. FEBS Lett. 579:5669-5674. PubMed
- Yao, F and M.A. Strauch (2005) Independent and Interchangeable Multimerization Domains of the AbrB, Abh and SpoVT Global Regulatory Proteins. J. Bacteriol. 187:6354-6362 PubMed
- Bobay, B.G., Mueller, G.A., Thompson, R.J., Venters, R.A., Murzin, A.G., Strauch, M.A. & J. Cavanagh (2006) NMR structure of AbhN and comparison with AbrBN: First Insights into the DNA-binding Promiscuity and Specificity of AbrB-like Transition-state Regulator Proteins. J. Biol. Chem. 281:21399-21409 PubMed
- Jordan S. Rietkötter E. Strauch MA. Kalamorz F. Butcher BG. Helmann JD. Mascher T. (2007) LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis. Microbiology. 153: 2530-2540. PubMed
- Strauch MA. Bobay BG. Cavanagh J. Yao F. Wilson A. Le Breton Y. (2007) Abh and AbrB control of Bacillus subtilis antimicrobial gene expression. J. of Bacteriol. 189:7720-7732. PubMed
- Hamze et al. (2009) Identification of genes required for different stages of dendritic swarming in Bacillus subtilis, with a novel role for phrC. Microbiology 155: 398-412. PubMed
- Strauch MA. (1995) AbrB modulates expression and catabolite repression of a Bacillus subtilis ribose transport operon. J Bacteriol. Dec;177(23):6727-31. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed