Difference between revisions of "QcrA"
(→Extended information on the protein) |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' menaquinol:cytochrome c oxidoreductase (iron-sulfur subunit), component of the cytochrome | + | * '''Description:''' Rieske factor, menaquinol:cytochrome c oxidoreductase (iron-sulfur subunit), component of the cytochrome bc1 complex<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 63: | Line 63: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 95: | Line 92: | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
** cell membrane {{PubMed|23880299}} | ** cell membrane {{PubMed|23880299}} | ||
| − | ** delivered to the membrane by the [[TatAY]]-[[TatCY]] [[protein secretion]] system {{PubMed|23256564}} | + | ** delivered to the membrane by the [[TatAY]]-[[TatCY]] [[protein secretion]] system, this depends on prior dusulfide bond formation and co-factor insertion {{PubMed|24652282,23256564}} |
=== Database entries === | === Database entries === | ||
| Line 150: | Line 147: | ||
<pubmed> 24140208 </pubmed> | <pubmed> 24140208 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>8631715,7592464, 23880299,12850135 12107147, 20817675 23256564 </pubmed> | + | <pubmed>8631715,7592464, 23880299,12850135 12107147, 20817675 23256564 24652282</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:17, 22 March 2014
- Description: Rieske factor, menaquinol:cytochrome c oxidoreductase (iron-sulfur subunit), component of the cytochrome bc1 complex
| Gene name | qcrA |
| Synonyms | bfcA, petC |
| Essential | no |
| Product | menaquinol:cytochrome c oxidoreductase (iron-sulfur subunit) |
| Function | respiration |
| Gene expression levels in SubtiExpress: qcrA | |
| Interactions involving this protein in SubtInteract: QcrA | |
| MW, pI | 18 kDa, 6.078 |
| Gene length, protein length | 501 bp, 167 aa |
| Immediate neighbours | qcrB, ypiF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 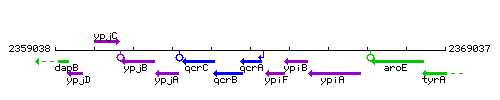
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
respiration, membrane proteins
This gene is a member of the following regulons
AbrB regulon, CcpA regulon, ResD regulon
The gene
Basic information
- Locus tag: BSU22560
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: accD/PCCB family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Cofactors: contains an iron-sulfur cluster
- Effectors of protein activity:
- Localization:
- cell membrane PubMed
- delivered to the membrane by the TatAY-TatCY protein secretion system, this depends on prior dusulfide bond formation and co-factor insertion PubMed
Database entries
- Structure:
- UniProt: P46911
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Vivianne J Goosens, Carmine G Monteferrante, Jan Maarten van Dijl
The Tat system of Gram-positive bacteria.
Biochim Biophys Acta: 2014, 1843(8);1698-706
[PubMed:24140208]
[WorldCat.org]
[DOI]
(P p)
Original publications