Difference between revisions of "SipW"
| Line 1: | Line 1: | ||
| − | * '''Description:''' signal peptidase I, required for biofilm formation <br/><br/> | + | * '''Description:''' signal peptidase I, processes [[TasA]] and [[TapA]], required for [[biofilm formation]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 20: | Line 20: | ||
|style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 570 bp, 190 aa | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 570 bp, 190 aa | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[tasA]]'', ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[tasA]]'', ''[[tapA]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB14394]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB14394]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | ||
| Line 56: | Line 56: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 64: | Line 62: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' Cleavage of hydrophobic, N-terminal signal or leader sequences from | + | * '''Catalyzed reaction/ biological activity:''' Cleavage of hydrophobic, N-terminal signal or leader sequences from [[TasA]] and [[TapA]] |
* '''Protein family:''' peptidase S26B family (according to Swiss-Prot) | * '''Protein family:''' peptidase S26B family (according to Swiss-Prot) | ||
| Line 84: | Line 82: | ||
* '''Interactions:''' | * '''Interactions:''' | ||
| − | * '''Localization:''' | + | * '''Localization:''' membrane |
=== Database entries === | === Database entries === | ||
| Line 100: | Line 98: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' ''[[ | + | * '''Operon:''' ''[[tapA]]-[[sipW]]-[[tasA]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/16430695 PubMed] |
* '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/16430695 PubMed] | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/16430695 PubMed] | ||
| Line 135: | Line 133: | ||
=References= | =References= | ||
| − | <pubmed>18047568,15175311,16430695,10368135,9694797,18430133,18647168,10464223,17720793, 20351052 10827084 </pubmed> | + | <pubmed>18047568,15175311,16430695,10368135,9694797,18430133,18647168,10464223,17720793, 20351052 10827084 21477127 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 07:44, 12 April 2011
- Description: signal peptidase I, processes TasA and TapA, required for biofilm formation
| Gene name | sipW |
| Synonyms | yqhE |
| Essential | no |
| Product | signal peptidase I |
| Function | biofilm formation |
| Regulation of this protein in SubtiPathways: Biofilm, Protein secretion | |
| MW, pI | 20 kDa, 5.494 |
| Gene length, protein length | 570 bp, 190 aa |
| Immediate neighbours | tasA, tapA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 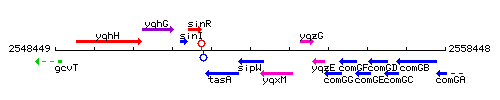
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
protein secretion, biofilm formation
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU24630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Cleavage of hydrophobic, N-terminal signal or leader sequences from TasA and TapA
- Protein family: peptidase S26B family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: membrane
Database entries
- Structure:
- UniProt: P54506
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References