Difference between revisions of "YcbD"
| Line 32: | Line 32: | ||
<br/><br/> | <br/><br/> | ||
| + | |||
| + | = [[Categories]] containing this gene/protein = | ||
| + | {{SubtiWiki category|[[utilization of specific carbon sources]]}} | ||
| + | |||
| + | = This gene is a member of the following [[regulons]] = | ||
| + | {{SubtiWiki regulon|[[YcbG regulon]]}} | ||
=The gene= | =The gene= | ||
| Line 51: | Line 57: | ||
| − | + | ||
| − | |||
=The protein= | =The protein= | ||
Revision as of 16:23, 8 December 2010
- Description: NADP(+)-dependent alpha-ketoglutaric semialdehyde dehydrogenase
| Gene name | ycbD |
| Synonyms | |
| Essential | no |
| Product | NADP(+)-dependent alpha-ketoglutaric semialdehyde dehydrogenase |
| Function | utilization of D-glucarate/galactarate |
| MW, pI | 52 kDa, 4.787 |
| Gene length, protein length | 1464 bp, 488 aa |
| Immediate neighbours | ycbC, ycbE |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 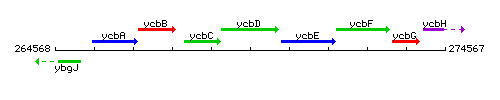
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02470
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: an aldehyde + NAD+ + H2O = an acid + NADH (according to Swiss-Prot)
- Protein family: aldehyde dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- UniProt: P42236
- KEGG entry: [3]
- E.C. number: 1.2.1.3
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Seiya Watanabe, Masaki Yamada, Iwao Ohtsu, Keisuke Makino
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, D-galactarate, and hydroxy-L-proline. Molecular and metabolic convergent evolution.
J Biol Chem: 2007, 282(9);6685-95
[PubMed:17202142]
[WorldCat.org]
[DOI]
(P p)
Shigeo Hosoya, Kunio Yamane, Michio Takeuchi, Tsutomu Sato
Identification and characterization of the Bacillus subtilis D-glucarate/galactarate utilization operon ycbCDEFGHJ.
FEMS Microbiol Lett: 2002, 210(2);193-9
[PubMed:12044674]
[WorldCat.org]
[DOI]
(P p)