Difference between revisions of "AprE"
| Line 127: | Line 127: | ||
<pubmed>3082852,2447063,2447062,19251843,18414485,6322190,12884008,15126467,1898931,1906467,2504584,18957862 11101663 12055299, </pubmed> | <pubmed>3082852,2447063,2447062,19251843,18414485,6322190,12884008,15126467,1898931,1906467,2504584,18957862 11101663 12055299, </pubmed> | ||
| + | |||
| + | [[Category:Protein-coding genes]] | ||
Revision as of 09:28, 21 July 2009
- Description: extracellular alkaline serine protease (subtilisin E)
| Gene name | aprE |
| Synonyms | sprE |
| Essential | no |
| Product | extracellular alkaline serine protease (subtilisin E)) |
| Function | protein degradation |
| MW, pI | 39 kDa, 9.342 |
| Gene length, protein length | 1143 bp, 381 aa |
| Immediate neighbours | yhfN, yhfO |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 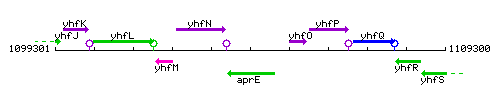
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU10300
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of proteins with broad specificity for peptide bonds, and a preference for a large uncharged residue in P1 (according to Swiss-Prot)
- Protein family: peptidase S8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: secreted (according to Swiss-Prot), extracellular (signal peptide) PubMed
Database entries
- Structure: 1SBC
- UniProt: P04189
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information: the mRNA is extremely stable (more than 25 min) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Sadanobu Abe, Ayako Yasumura, Teruo Tanaka
Regulation of Bacillus subtilis aprE expression by glnA through inhibition of scoC and sigma(D)-dependent degR expression.
J Bacteriol: 2009, 191(9);3050-8
[PubMed:19251843]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Jan-Willem Veening, Oleg A Igoshin, Robyn T Eijlander, Reindert Nijland, Leendert W Hamoen, Oscar P Kuipers
Transient heterogeneity in extracellular protease production by Bacillus subtilis.
Mol Syst Biol: 2008, 4;184
[PubMed:18414485]
[WorldCat.org]
[DOI]
(I p)
Mitsuo Ogura, Atsushi Matsuzawa, Hirofumi Yoshikawa, Teruo Tanaka
Bacillus subtilis SalA (YbaL) negatively regulates expression of scoC, which encodes the repressor for the alkaline exoprotease gene, aprE.
J Bacteriol: 2004, 186(10);3056-64
[PubMed:15126467]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
Gustav Hambraeus, Kaisa Karhumaa, Blanka Rutberg
A 5' stem-loop and ribosome binding but not translation are important for the stability of Bacillus subtilis aprE leader mRNA.
Microbiology (Reading): 2002, 148(Pt 6);1795-1803
[PubMed:12055299]
[WorldCat.org]
[DOI]
(P p)
Gustav Hambraeus, Martin Persson, Blanka Rutberg
The aprE leader is a determinant of extreme mRNA stability in Bacillus subtilis.
Microbiology (Reading): 2000, 146 Pt 12;3051-3059
[PubMed:11101663]
[WorldCat.org]
[DOI]
(P p)
P T Kallio, J E Fagelson, J A Hoch, M A Strauch
The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein.
J Biol Chem: 1991, 266(20);13411-7
[PubMed:1906467]
[WorldCat.org]
(P p)
N K Gaur, J Oppenheim, I Smith
The Bacillus subtilis sin gene, a regulator of alternate developmental processes, codes for a DNA-binding protein.
J Bacteriol: 1991, 173(2);678-86
[PubMed:1898931]
[WorldCat.org]
[DOI]
(P p)
M A Strauch, G B Spiegelman, M Perego, W C Johnson, D Burbulys, J A Hoch
The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein.
EMBO J: 1989, 8(5);1615-21
[PubMed:2504584]
[WorldCat.org]
[DOI]
(P p)
D J Henner, E Ferrari, M Perego, J A Hoch
Location of the targets of the hpr-97, sacU32(Hy), and sacQ36(Hy) mutations in upstream regions of the subtilisin promoter.
J Bacteriol: 1988, 170(1);296-300
[PubMed:2447063]
[WorldCat.org]
[DOI]
(P p)
E Ferrari, D J Henner, M Perego, J A Hoch
Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants.
J Bacteriol: 1988, 170(1);289-95
[PubMed:2447062]
[WorldCat.org]
[DOI]
(P p)
E Ferrari, S M Howard, J A Hoch
Effect of stage 0 sporulation mutations on subtilisin expression.
J Bacteriol: 1986, 166(1);173-9
[PubMed:3082852]
[WorldCat.org]
[DOI]
(P p)
S L Wong, C W Price, D S Goldfarb, R H Doi
The subtilisin E gene of Bacillus subtilis is transcribed from a sigma 37 promoter in vivo.
Proc Natl Acad Sci U S A: 1984, 81(4);1184-8
[PubMed:6322190]
[WorldCat.org]
[DOI]
(P p)