Difference between revisions of "PtkA"
(→Basic information/ Evolution) |
|||
| Line 80: | Line 80: | ||
* '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2VED 2VED] (CapB, the homolog in ''Staphylococcus aureus'') | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2VED 2VED] (CapB, the homolog in ''Staphylococcus aureus'') | ||
| − | * ''' | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/P96716 P96716] |
* '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU36250] | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU36250] | ||
Revision as of 14:53, 20 July 2009
- Description: protein tyrosine kinase
| Gene name | ptkA |
| Synonyms | ywqD |
| Essential | no |
| Product | protein tyrosine kinase |
| Function | protein phosphorylation |
| MW, pI | 25 kDa, 9.628 |
| Gene length, protein length | 711 bp, 237 aa |
| Immediate neighbours | ptpZ, tkmA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 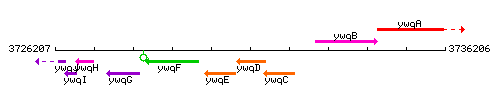
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU36250
Phenotypes of a mutant
Accumulation of extra chromosome equivalents PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + a [protein]-L-tyrosine = ADP + a [protein]-L-tyrosine phosphate (according to Swiss-Prot), autophosphorylation, phosphorylation of YwqF, TuaD, Ssb, SsbB
- Protein family: cpsD/capB family (according to Swiss-Prot), BY-kinase
- Paralogous protein(s): EpsB
Extended information on the protein
- Kinetic information:
- Domains: single BY-kinase domain
- Modification: autophosphorylation at residues Y225, Y227 and Y228 (primary site)
- Cofactor(s): ATP
- Effectors of protein activity: TkmA - transmembrane modulator, activates PtkA autophosphorylation and substrate phosphorylation PubMed
- Localization:
Database entries
- Structure: 2VED (CapB, the homolog in Staphylococcus aureus)
- UniProt: P96716
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: KO strain created with pMUTIN-2, available from Ivan Mijakovic
- Expression vector: pQE-30, N-terminally 6xHis-tagged, available from Ivan Mijakovic
- lacZ fusion: in a KO strain created with pMUTIN-2, available from Ivan Mijakovic
- GFP fusion: CFP-fusion, available from Ivan Mijakovic
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Ivan Mijakovic, Thiverval-Grignon, France
Your additional remarks
References
Dina Petranovic, Christophe Grangeasse, Boris Macek, Mohammad Abdillatef, Virginie Gueguen-Chaignon, Sylvie Nessler, Josef Deutscher, Ivan Mijakovic
Activation of Bacillus subtilis Ugd by the BY-kinase PtkA proceeds via phosphorylation of its residue tyrosine 70.
J Mol Microbiol Biotechnol: 2009, 17(2);83-9
[PubMed:19258708]
[WorldCat.org]
[DOI]
(I p)
Dina Petranovic, Ole Michelsen, Ksenija Zahradka, Catarina Silva, Mirjana Petranovic, Peter Ruhdal Jensen, Ivan Mijakovic
Bacillus subtilis strain deficient for the protein-tyrosine kinase PtkA exhibits impaired DNA replication.
Mol Microbiol: 2007, 63(6);1797-805
[PubMed:17367396]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Lucia Musumeci, Lutz Tautz, Dina Petranovic, Robert A Edwards, Peter Ruhdal Jensen, Tomas Mustelin, Josef Deutscher, Nunzio Bottini
In vitro characterization of the Bacillus subtilis protein tyrosine phosphatase YwqE.
J Bacteriol: 2005, 187(10);3384-90
[PubMed:15866923]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Dina Petranovic, Josef Deutscher
How tyrosine phosphorylation affects the UDP-glucose dehydrogenase activity of Bacillus subtilis YwqF.
J Mol Microbiol Biotechnol: 2004, 8(1);19-25
[PubMed:15741737]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Sandrine Poncet, Grégory Boël, Alain Mazé, Sylvie Gillet, Emmanuel Jamet, Paulette Decottignies, Christophe Grangeasse, Patricia Doublet, Pierre Le Maréchal, Josef Deutscher
Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases.
EMBO J: 2003, 22(18);4709-18
[PubMed:12970183]
[WorldCat.org]
[DOI]
(P p)