Difference between revisions of "RapK"
| Line 65: | Line 65: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 144: | Line 142: | ||
=References= | =References= | ||
| − | <pubmed> 11466295 16816200 20817675</pubmed> | + | <pubmed> 11466295 16816200 20817675 25225273 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:11, 19 September 2014
- Description: response regulator aspartate phosphatase, controls ComA activity
| Gene name | rapK |
| Synonyms | yobG |
| Essential | no |
| Product | response regulator aspartate phosphatase |
| Function | control of ComA activity |
| Gene expression levels in SubtiExpress: rapK | |
| Interactions involving this protein in SubtInteract: RapK | |
| MW, pI | 43 kDa, 5.06 |
| Gene length, protein length | 1113 bp, 371 aa |
| Immediate neighbours | yozZ, phrK |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 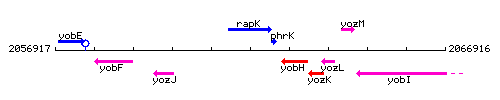
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed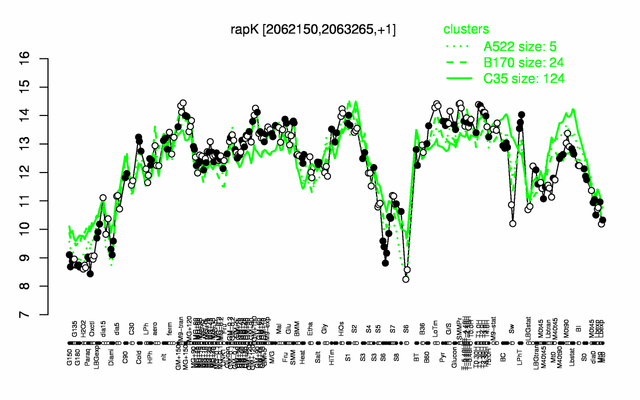
| |
Contents
Categories containing this gene/protein
genetic competence, protein modification, transcription factors and their control, quorum sensing
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU18910
Phenotypes of a mutant
Database entries
- BsubCyc: BSU18910
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
Database entries
- BsubCyc: BSU18910
- Structure:
- UniProt: O34930
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 318 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Cláudia R Serra, Ashlee M Earl, Teresa M Barbosa, Roberto Kolter, Adriano O Henriques
Sporulation during growth in a gut isolate of Bacillus subtilis.
J Bacteriol: 2014, 196(23);4184-96
[PubMed:25225273]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Jennifer M Auchtung, Catherine A Lee, Alan D Grossman
Modulation of the ComA-dependent quorum response in Bacillus subtilis by multiple Rap proteins and Phr peptides.
J Bacteriol: 2006, 188(14);5273-85
[PubMed:16816200]
[WorldCat.org]
[DOI]
(P p)
R S McQuade, N Comella, A D Grossman
Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis.
J Bacteriol: 2001, 183(16);4905-9
[PubMed:11466295]
[WorldCat.org]
[DOI]
(P p)