Difference between revisions of "PfkA"
(→Phenotypes of a mutant) |
|||
| Line 102: | Line 102: | ||
=Biological materials = | =Biological materials = | ||
| + | |||
| + | * '''Mutant:''' | ||
| + | |||
| + | * '''Expression vector:''' | ||
| + | |||
| + | * '''lacZ fusion:''' | ||
| + | |||
| + | * '''GFP fusion:''' | ||
| + | |||
| + | * '''Antibody:''' | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
Revision as of 12:44, 14 January 2009
- Description: phosphofructokinase, glycolytic enzyme
| Gene name | pfkA |
| Synonyms | pfk |
| Essential | yes |
| Product | 6-phosphofructokinase |
| Function | catabolic enzyme in glycolysis |
| MW, pI | 34,1 kDa, 6.14 |
| Gene length, protein length | 957 bp, 319 amino acids |
| Immediate neighbours | accA, pyk |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 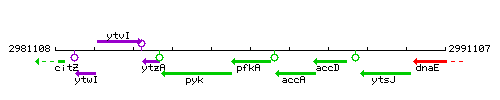
| |
Contents
The gene
Basic information
- Coordinates: 2985630 - 2986586
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + D-fructose 6-phosphate = ADP + D-fructose 1,6-bisphosphate
- Protein family: phosphofructokinase family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- 3 x nucleotide binding domain (ATP) (21–25), (154–158), (171–187)
- Modification:
- Cofactor(s): ATP
- Effectors of protein activity:
- Interactions: Pyk-PfkA
- Localization: cytoplasm PubMed
Database entries
- Structure: Geobacillus stearothermophilus NCBI, Mutant form, complex with fructose-6-phosphate Geobacillus stearothermophilus NCBI
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: [5]
Additional information
Expression and regulation
- Operon: pfkA pyk
- Sigma factor:
- Regulation: twofold induced by glucose
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Commichau, F. M., Rothe, F. M., Herzberg, C., Wagner, E., Hellwig, D., Lehnik-Habrink, M., Hammer, E., Völker, U. & Stülke, J. Novel activities of glycolytic enzymes in Bacillus subtilis: Interactions with essential proteins involved in mRNA processing. subm.
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.PubMed