Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' ATP-dependent Clp protease | + | * '''Description:''' ATP-dependent Clp protease proteolytic subunit (class III heat-shock protein) <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''clpP'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''yvdN '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || ATP-dependent Clp protease | + | |style="background:#ABCDEF;" align="center"| '''Product''' || ATP-dependent Clp protease proteolytic subunit |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || protein degradation | |style="background:#ABCDEF;" align="center"|'''Function''' || protein degradation | ||
| Line 16: | Line 16: | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/stress_response.html Stress]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/stress_response.html Stress]''' | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 21 kDa, 5.008 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 591 bp, 197 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[trnQ-Arg]]'', ''[[pgcM]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB15459]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:clpP_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 31: | Line 31: | ||
__TOC__ | __TOC__ | ||
| − | + | <br/><br/> | |
=The gene= | =The gene= | ||
| Line 37: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU34540 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| Line 43: | Line 43: | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/clpP.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG19016] |
=== Additional information=== | === Additional information=== | ||
| Line 54: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' Hydrolysis of proteins to small peptides in the presence of ATP and magnesium (according to Swiss-Prot) endopeptidase/proteolysis |
| − | * '''Protein family:''' | + | * '''Protein family:''' peptidase S14 family (according to Swiss-Prot) ClpP (IPR001907) [http://www.ebi.ac.uk/interpro/DisplayIproEntry?ac=IPR001907 InterPro], (PF00574) [http://pfam.sanger.ac.uk/family?acc=PF00574 PFAM] |
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 64: | Line 64: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''Domains:''' |
* '''Modification:''' | * '''Modification:''' | ||
| Line 72: | Line 72: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' [[ClpE]]-[[ClpP]] | + | * '''Interactions:''' [[ClpE]]-[[ClpP]], [[ClpC]]-[[ClpP]], [[ClpX]]-[[ClpP]] |
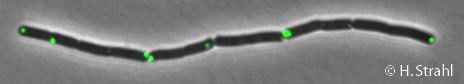
| − | * '''Localization:''' | + | * '''Localization:''' cytoplasm (according to Swiss-Prot), cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heatshock, colocalization with [[ClpX]], [[ClpC]] and [[ClpE]] [http://www.ncbi.nlm.nih.gov/pubmed/18786145 Pubmed] |
| − | [[File: | + | |
| + | [[File:ClpP.jpg ]] | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' Two homologue structures resolved [http://www.rcsb.org/pdb/explore/explore.do?structureId=1TYF 1TYF], [http://www.rcsb.org/pdb/explore/explore.do?structureId=1Y7O 1Y7O], structural model of ''B. subtilis'' [[ClpP]] available from [http://subtiwiki.uni-goettingen.de/wiki/index.php/User:Hstrahl hstrahl] |
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P80244 P80244] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU34540] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' [http://www.expasy.org/enzyme/3.4.21.92 3.4.21.92] |
=== Additional information=== | === Additional information=== | ||
| Line 93: | Line 94: | ||
* '''Operon:''' | * '''Operon:''' | ||
| − | * '''[[Sigma factor]]:''' | + | * '''[[Sigma factor]]:''' [[SigB]] [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed] |
| − | * '''Regulation:''' induced by heat ([[CtsR]]) [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed] | + | * '''Regulation:''' induced by stress ([[SigB]]) [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed], induced by heat ([[CtsR]]) [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed] |
* '''Regulatory mechanism:''' [[CtsR]]: transcription repression [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed1], [http://www.ncbi.nlm.nih.gov/pubmed/11179229 PubMed2], [http://www.ncbi.nlm.nih.gov/pubmed/16163393 PubMed3], [http://www.ncbi.nlm.nih.gov/pubmed/17380125 PubMed4] | * '''Regulatory mechanism:''' [[CtsR]]: transcription repression [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed1], [http://www.ncbi.nlm.nih.gov/pubmed/11179229 PubMed2], [http://www.ncbi.nlm.nih.gov/pubmed/16163393 PubMed3], [http://www.ncbi.nlm.nih.gov/pubmed/17380125 PubMed4] | ||
| Line 103: | Line 104: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' '' | + | * '''Mutant:''' ''clpP::spec'' and ''clpP::cat'' available from the [http://subtiwiki.uni-goettingen.de/wiki/index.php/Leendert_Hamoen Hamoen]] Lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 109: | Line 110: | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| − | * '''GFP fusion:''' C-terminal | + | * '''GFP fusion:''' C-terminal GFP fusions (both single copy and as 2th copy in ''amyE'' locus, also as CFP and YFP variants) available from the [http://subtiwiki.uni-goettingen.de/wiki/index.php/Leendert_Hamoen Hamoen]] Lab |
* '''two-hybrid system:''' | * '''two-hybrid system:''' | ||
| Line 121: | Line 122: | ||
=References= | =References= | ||
| + | |||
| + | <pubmed>11544224 14763982 9643546, </pubmed> | ||
| + | # Petersohn et al. (2001) Global Analysis of the General Stress Response of ''Bacillus subtilis''. ''J Bacteriol.'' '''183:''' 5617-5631 [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed] | ||
| + | # Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. [http://www.ncbi.nlm.nih.gov/sites/entrez/14763982 PubMed] | ||
| + | # Gerth, U., Krüger, E., Derré, I., Msadek, T. and Hecker, M. 1998. Stress induction of the [[Bacillus subtilis clpP]] gene encoding a homologue of the proteolytic component of the ClpP protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28: 787-802. [http://www.ncbi.nlm.nih.gov/sites/entrez/9643546 PubMed] | ||
# Kirstein, J., Strahl, H., Molière, N., Hamoen, LW., Turgay K. (2008) Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 70:682-94. [http://www.ncbi.nlm.nih.gov/pubmed/18786145 Pubmed] | # Kirstein, J., Strahl, H., Molière, N., Hamoen, LW., Turgay K. (2008) Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 70:682-94. [http://www.ncbi.nlm.nih.gov/pubmed/18786145 Pubmed] | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 10:42, 11 June 2009
- Description: ATP-dependent Clp protease proteolytic subunit (class III heat-shock protein)
| Gene name | clpP |
| Synonyms | yvdN |
| Essential | no |
| Product | ATP-dependent Clp protease proteolytic subunit |
| Function | protein degradation |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 21 kDa, 5.008 |
| Gene length, protein length | 591 bp, 197 aa |
| Immediate neighbours | trnQ-Arg, pgcM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 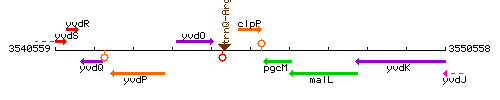
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU34540
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of proteins to small peptides in the presence of ATP and magnesium (according to Swiss-Prot) endopeptidase/proteolysis
- Protein family: peptidase S14 family (according to Swiss-Prot) ClpP (IPR001907) InterPro, (PF00574) PFAM
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot), cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heatshock, colocalization with ClpX, ClpC and ClpE Pubmed
Database entries
- Structure: Two homologue structures resolved 1TYF, 1Y7O, structural model of B. subtilis ClpP available from hstrahl
- Swiss prot entry: P80244
- KEGG entry: [3]
- E.C. number: 3.4.21.92
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant: clpP::spec and clpP::cat available from the Hamoen] Lab
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (both single copy and as 2th copy in amyE locus, also as CFP and YFP variants) available from the Hamoen] Lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
- Petersohn et al. (2001) Global Analysis of the General Stress Response of Bacillus subtilis. J Bacteriol. 183: 5617-5631 PubMed
- Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. PubMed
- Gerth, U., Krüger, E., Derré, I., Msadek, T. and Hecker, M. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the ClpP protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28: 787-802. PubMed
- Kirstein, J., Strahl, H., Molière, N., Hamoen, LW., Turgay K. (2008) Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 70:682-94. Pubmed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed