Difference between revisions of "GltC"
| Line 37: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * ''' | + | * '''Locus tag:''' |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
Revision as of 12:35, 2 June 2009
- Description: Transcriptional activator of the gltAB operon. Activates expression of the operon in the absence of arginine.
| Gene name | gltC |
| Synonyms | |
| Essential | No |
| Product | transcriptional regulator (LysR family) |
| Function | positive regulation of the glutamate synthase operon (gltAB) |
| MW, pI | 33.9 kDa, 5.62 |
| Gene length, protein length | 900 bp, 300 amino acids |
| Immediate neighbours | gltA, proJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 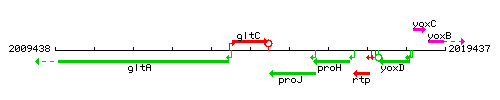
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag:
Phenotypes of a mutant
gltC mutants are auxotrophic for glutamate.
Database entries
- DBTBS entry: [1]
- SubtiList entry:[2]
Additional information
The protein
Basic information/ Evolution
- Protein family: glutamate synthase family (according to Swiss-Prot) LysR-type transcription regulator PubMed
- Paralogous protein(s): none, but there are 19 members of the LysR family in B. subtilis
Extended information on the protein
- Kinetic information:
- Domains: DNA-binding helix-turn-helix motif: AA 18 ... 37
- Modification:
- Cofactor(s):
- Effectors of protein activity: 2-oxoglutarate stimulates transcription activation, glutamate inhibits transcription activation PubMed
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P20668
- KEGG entry: BSU18460
Additional information
Expression and regulation
- Operon: gltC
- Sigma factor: SigA
- Regulation: autoregulation by GltC
- Regulatory mechanism: autorepression
- Database entries: DBTBS
- Additional information:
Biological materials
- Mutant: GP344 (erm), GP738 (spc) (available in Stülke lab)
- Expression vector: pGP903 (in pWH844, N-terminal His-tag), pGP951 (C-terminal Strep-tag) (available in Stülke lab)
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody: available in Stülke lab
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Yoshida K, et al. (2003) Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol Microbiol 49(1): 157-65. PubMed
- Belitsky, B. R., and Sonenshein, A. L. (1995) Mutations in GltC that increase Bacillus subtilis gltA expression. J Bacteriol 177: 5696-5700.PubMed
- Belitsky, B. R., and Sonenshein, A. L. (2004) Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase. J Bacteriol 186: 3399-3407.PubMed
- Bohannon, D. E., and Sonenshein, A. L. (1989) Positive regulation of glutamate biosynthesis in Bacillus subtilis. J Bacteriol 171: 4718-4727.PubMed
- Commichau, F. M., Wacker, I., Schleider, J., Blencke, H.-M., Reif, I., Tripal, P., and Stülke, J. (2007) Characterization of Bacillus subtilis mutants with carbon source-independent glutamate biosynthesis. J Mol Microbiol Biotechnol 12: 106-113. PubMed
- Commichau, F. M., Herzberg, C., Tripal, P., Valerius, O., and Stülke, J. (2007) A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: The glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol Microbiol 65: 642-654. PubMed
- Picossi, S., Belitsky, B. R., and Sonenshein, A. L. (2007) Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC. J Mol Biol 365: 1298-1313. PubMed
- Wacker, I., Ludwig, H., Reif, I., Blencke, H. M., Detsch, C., and Stülke, J. (2003) The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149: 3001-3009.PubMed
- Herzberg, C., Flórez Weidinger, L. A., Dörrbecker, B., Hübner, S., Stülke, J. & Commichau, F. M. (2007) SPINE: A method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7: 4032-4035. PubMed
- Schell, M. A. (1993). Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol 47, 597-626. PubMed