Difference between revisions of "LiaI"
| Line 1: | Line 1: | ||
| − | * '''Description:''' resistance against oxidative stress and cell wall antibiotics, | + | * '''Description:''' resistance against oxidative stress and cell wall antibiotics, membrane anchor for [[LiaH]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || membrane anchor for [[LiaH]] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || resistance against oxidative stress and cell wall antibiotics | |style="background:#ABCDEF;" align="center"|'''Function''' || resistance against oxidative stress and cell wall antibiotics | ||
| Line 61: | Line 61: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 88: | Line 85: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| + | ** [[LiaI]]-[[LiaH]] {{PubMed|24666271}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| − | ** cell membrane | + | ** cell membrane {{PubMed|24666271}} |
| + | ** forms highly dynamic membrane-associated foci under non-inducing conditions {{PubMed|24666271}} | ||
| + | ** co-localizes with [[LiaH]] in distinct static spots at the cytoplasma membrane under stress conditions {{PubMed|24666271}} | ||
=== Database entries === | === Database entries === | ||
| Line 145: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed>19164152,15273097,17660417,17600057,20057163 ,16816187,15101989,17660417,12850135 16816187, 20639339 20817675 ,22092710</pubmed> | + | <pubmed>19164152,15273097,17660417,17600057,20057163 ,16816187,15101989,17660417,12850135 16816187, 20639339 20817675 ,22092710 24666271 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:09, 10 October 2014
- Description: resistance against oxidative stress and cell wall antibiotics, membrane anchor for LiaH
| Gene name | liaI |
| Synonyms | yvqI |
| Essential | no |
| Product | membrane anchor for LiaH |
| Function | resistance against oxidative stress and cell wall antibiotics |
| Gene expression levels in SubtiExpress: liaI | |
| MW, pI | 13 kDa, 9.303 |
| Gene length, protein length | 378 bp, 126 aa |
| Immediate neighbours | liaH, yvqJ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 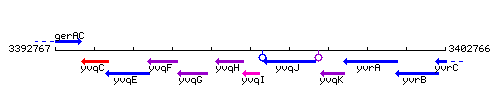
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed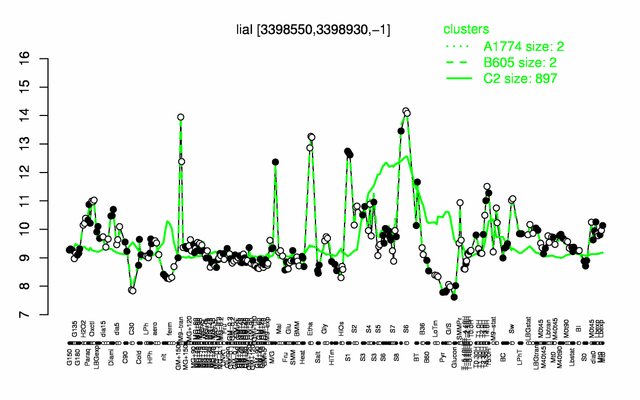
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress, resistance against toxins/ antibiotics, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33130
Phenotypes of a mutant
Database entries
- BsubCyc: BSU33130
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU33130
- Structure:
- UniProt: O32202
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References