Difference between revisions of "XynC"
(→Original publications) |
|||
| (15 intermediate revisions by 3 users not shown) | |||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || xylan degradation | |style="background:#ABCDEF;" align="center"|'''Function''' || xylan degradation | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU18150 xynC] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 9.078 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 9.078 | ||
| Line 20: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ynfE]]'', ''[[xynD]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ynfE]]'', ''[[xynD]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU18150 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU18150 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU18150 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ynfF_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ynfF_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=xynC_1942714_1943982_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:xynC_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU18150]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| − | + | <br/><br/><br/><br/> | |
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
<br/><br/> | <br/><br/> | ||
| Line 35: | Line 41: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| − | + | {{SubtiWiki regulon|[[AbrB regulon]]}} | |
=The gene= | =The gene= | ||
| Line 46: | Line 52: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU18150&redirect=T BSU18150] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 78: | Line 85: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''[[SubtInteract|Interactions]]:''' |
| − | * '''Localization:''' | + | * '''[[Localization]]:''' |
| + | ** extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU18150&redirect=T BSU18150] | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=3GTN 3GTN] | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=3GTN 3GTN] {{PubMed|19407387,21256135}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/Q45070 Q45070] | * '''UniProt:''' [http://www.uniprot.org/uniprot/Q45070 Q45070] | ||
| Line 96: | Line 105: | ||
=Expression and regulation= | =Expression and regulation= | ||
* '''Operon:''' ''[[xynD]]-[[xynC]]'' {{PubMed|20817675}} | * '''Operon:''' ''[[xynD]]-[[xynC]]'' {{PubMed|20817675}} | ||
| + | |||
| + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=xynC_1942714_1943982_-1 xynC] {{PubMed|22383849}} | ||
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| Line 104: | Line 115: | ||
** [[AbrB]]: transcription repression {{PubMed|20817675}} | ** [[AbrB]]: transcription repression {{PubMed|20817675}} | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| Line 128: | Line 139: | ||
<pubmed>20735481 </pubmed> | <pubmed>20735481 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | + | <pubmed>19407387, 18957862, 17028274 20817675,21256135 24271172 </pubmed> | |
| − | <pubmed>19407387 | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Latest revision as of 13:51, 2 April 2014
- Description: endo-xylanase, preference for methylglucurono-xylan
| Gene name | xynC |
| Synonyms | ynfF |
| Essential | no |
| Product | endo-xylanase |
| Function | xylan degradation |
| Gene expression levels in SubtiExpress: xynC | |
| MW, pI | 47 kDa, 9.078 |
| Gene length, protein length | 1266 bp, 422 aa |
| Immediate neighbours | ynfE, xynD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 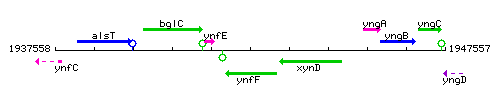
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU18150
Phenotypes of a mutant
Database entries
- BsubCyc: BSU18150
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Endohydrolysis of (1->4)-beta-D-xylosyl links in some glucuronoarabinoxylans (according to Swiss-Prot)
- Protein family: glycosyl hydrolase 30 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- extracellular (signal peptide) PubMed
Database entries
- BsubCyc: BSU18150
- UniProt: Q45070
- KEGG entry: [2]
- E.C. number: 3.2.1.136
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications