Difference between revisions of "TepA"
| Line 1: | Line 1: | ||
| − | * '''Description:''' [[germination]] protease, | + | * '''Description:''' orphan ClpP-like [[germination]] protease, contributes to SASP degradation<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 40: | Line 40: | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| − | {{SubtiWiki category|[[ | + | {{SubtiWiki category|[[proteolysis]]}}, |
| − | {{SubtiWiki category|[[ | + | {{SubtiWiki category|[[germination]]}} |
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| − | {{SubtiWiki regulon|[[SigG regulon]]}} | + | {{SubtiWiki regulon|[[SigG regulon]]}}, |
| + | {{SubtiWiki regulon|[[SpoVT regulon]]}}, | ||
=The gene= | =The gene= | ||
| Line 70: | Line 71: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| + | ** degradation of SASPs in germinating spores {{PubMed|23927687}} | ||
* '''Protein family:''' TepA subfamily (according to Swiss-Prot) | * '''Protein family:''' TepA subfamily (according to Swiss-Prot) | ||
| Line 86: | Line 88: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| + | ** activity requires [[YlzJ]] {{PubMed|23927687}} | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| Line 113: | Line 116: | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| − | ** expressed late during [[sporulation]] in the forespore ([[SigG]]) {{PubMed|23123912}} | + | ** expressed late during [[sporulation]] in the forespore ([[SigG]], [[SpoVT]]) {{PubMed|23123912}} |
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[SpoVT]]: transcription repression {{PubMed|23123912}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 14:32, 12 August 2013
- Description: orphan ClpP-like germination protease, contributes to SASP degradation
| Gene name | tepA |
| Synonyms | ymfB, ylxI |
| Essential | no |
| Product | germination protease |
| Function | degradation of SASPs |
| Gene expression levels in SubtiExpress: tepA | |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 24 kDa, 5.575 |
| Gene length, protein length | 669 bp, 223 aa |
| Immediate neighbours | rnjB, ylzJ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 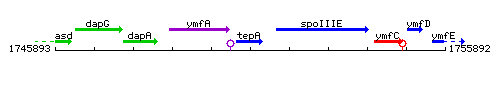
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16790
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- degradation of SASPs in germinating spores PubMed
- Protein family: TepA subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: Q99171
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- expressed late during sporulation in the forespore (SigG, SpoVT) PubMed
- Additional information:
Biological materials
- Mutant: GP1120 (spc), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References