Difference between revisions of "GltA"
| Line 138: | Line 138: | ||
<pubmed>12823818,,11029411,12850135 7559360 15150225 2548995 17183217, 17608797,17134717,14523131,12823818, 18326565,17981983,18763711 </pubmed> | <pubmed>12823818,,11029411,12850135 7559360 15150225 2548995 17183217, 17608797,17134717,14523131,12823818, 18326565,17981983,18763711 </pubmed> | ||
| + | |||
| + | [[Category:Protein-coding genes]] | ||
Revision as of 11:04, 21 July 2009
- Description: large subunit of glutamate synthase
| Gene name | gltA |
| Synonyms | |
| Essential | no |
| Product | glutamate synthase (large subunit) |
| Function | glutamate biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 168 kDa, 5.47 |
| Gene length, protein length | 4560 bp, 1520 amino acids |
| Immediate neighbours | gltC, gltB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 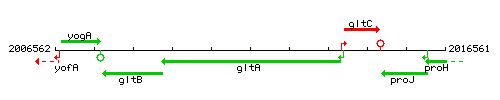
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU18450
Phenotypes of a mutant
auxotrophic for glutamate
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 L-glutamate + NADP+ = L-glutamine + 2-oxoglutarate + NADPH (according to Swiss-Prot) 2 L-glutamate + NADP(+) <=> L-glutamine + 2-oxoglutarate + NADPH
- Protein family: glutamate synthase family (according to Swiss-Prot) glutamate synthase family
- Paralogous protein(s): YerD
Extended information on the protein
- Kinetic information:
- Domains:
- Glutamine amidotransferase type-2 domain (22-415)
- Nucleotide binding domain (1060-1112)
- Modification:
- Cofactor(s): 3Fe-4S, FAD, FMN
- Effectors of protein activity:
- Interactions:
- Localization: membrane associated PubMed, cytoplasm
Database entries
- Structure:
- UniProt: P39812
- KEGG entry: [3]
- E.C. number: 1.4.1.13 3 1.4.1.13]
Additional information
subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation: expression activated by glucose (11 fold) PubMed, induced by sugar PubMed, repressed by arginine PubMed, ammonium required PubMed
- Regulatory mechanism: Activator: GltC PubMed; Repressor: TnrA PubMed, pos. regulated by a mutant form of GltR PubMed
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant: GP807 (del gltAB::tet), GP222 (gltA under the control of p-xyl), available in Stülke lab
- Expression vector:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References