SafA
- Description: morphogenetic protein associated with SpoVID, major organizer of the inner spore coat
| Gene name | safA |
| Synonyms | yrbA |
| Essential | no |
| Product | morphogenetic protein |
| Function | spore coat formation |
| Gene expression levels in SubtiExpress: safA | |
| Interactions involving this protein in SubtInteract: SafA | |
| MW, pI | 43 kDa, 5.753 |
| Gene length, protein length | 1161 bp, 387 aa |
| Immediate neighbours | coxA, nadA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 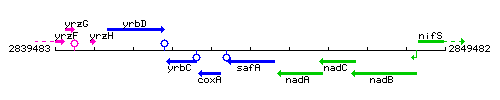
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed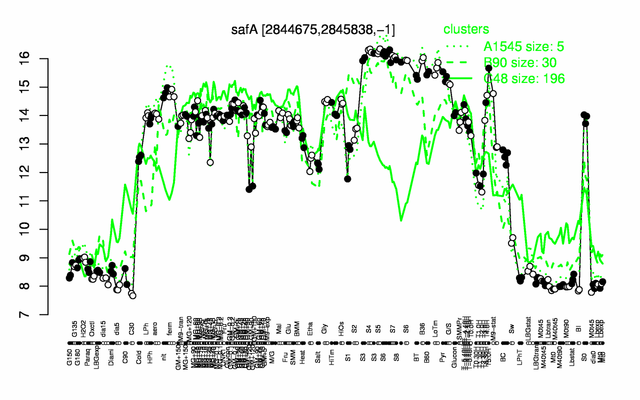
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
SafA-dependent proteins of the inner spore coat
- CotD, CotP, CotT, CwlJ, OxdD, YaaH, YeeK, YisY, YhjR, YjqC, YmaG, YsnD, YsxE, YutH, YuzC, YxeE, YybI PubMed
The gene
Basic information
- Locus tag: BSU27840
Phenotypes of a mutant
- mis-assembly of the inner spore coat PubMed
Database entries
- BsubCyc: BSU27840
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- contains a N-acetylglucosamine-polymer-binding LysM domain at the N-terminus PubMed
- Modification:
- Effectors of protein activity:
- Localization:
- inner spore coat PubMed
Database entries
- BsubCyc: BSU27840
- Structure:
- UniProt: O32062
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Adriano Henriques, Lisbon, Portugal homepage
- Charles Moran, Emory University, NC, USA homepage
Your additional remarks
References
Reviews
Peter T McKenney, Adam Driks, Patrick Eichenberger
The Bacillus subtilis endospore: assembly and functions of the multilayered coat.
Nat Rev Microbiol: 2013, 11(1);33-44
[PubMed:23202530]
[WorldCat.org]
[DOI]
(I p)
Peter Setlow
Dynamics of the assembly of a complex macromolecular structure--the coat of spores of the bacterium Bacillus subtilis.
Mol Microbiol: 2012, 83(2);241-4
[PubMed:22192522]
[WorldCat.org]
[DOI]
(I p)
Girbe Buist, Anton Steen, Jan Kok, Oscar P Kuipers
LysM, a widely distributed protein motif for binding to (peptido)glycans.
Mol Microbiol: 2008, 68(4);838-47
[PubMed:18430080]
[WorldCat.org]
[DOI]
(I p)
Original publications