YfiT
Revision as of 12:48, 16 May 2013 by 134.76.70.252 (talk)
- Description: bacillithiol S-transferase
| Gene name | yfiT |
| Synonyms | |
| Essential | no |
| Product | bacillithiol S-transferase |
| Function | detoxification |
| Gene expression levels in SubtiExpress: yfiT | |
| MW, pI | 20 kDa, 6.612 |
| Gene length, protein length | 534 bp, 178 aa |
| Immediate neighbours | yfiS, yfiU |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 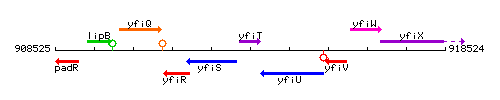
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU08390
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: metal hydrolase yfiT family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Ni(2+) PubMed
- Effectors of protein activity:
- Interactions:
- homodimer PubMed
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1RXQ
- UniProt: O31562
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: yfiT (according to DBTBS)
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Gerald L Newton, Stephan S Leung, Judy I Wakabayashi, Mamta Rawat, Robert C Fahey
The DinB superfamily includes novel mycothiol, bacillithiol, and glutathione S-transferases.
Biochemistry: 2011, 50(49);10751-60
[PubMed:22059487]
[WorldCat.org]
[DOI]
(I p)
Shyamala S Rajan, Xiaojing Yang, Ludmilla Shuvalova, Frank Collart, Wayne F Anderson
YfiT from Bacillus subtilis is a probable metal-dependent hydrolase with an unusual four-helix bundle topology.
Biochemistry: 2004, 43(49);15472-9
[PubMed:15581359]
[WorldCat.org]
[DOI]
(P p)