TagF
- Description: CDP-glycerol:polyglycerol phosphate glycero-phosphotransferase
| Gene name | tagF |
| Synonyms | rodC |
| Essential | yes PubMed |
| Product | CDP-glycerol:polyglycerol phosphate glycero-phosphotransferase |
| Function | biosynthesis of teichoic acid |
| Gene expression levels in SubtiExpress: tagF | |
| MW, pI | 87 kDa, 9.4 |
| Gene length, protein length | 2238 bp, 746 aa |
| Immediate neighbours | tagG, tagE |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed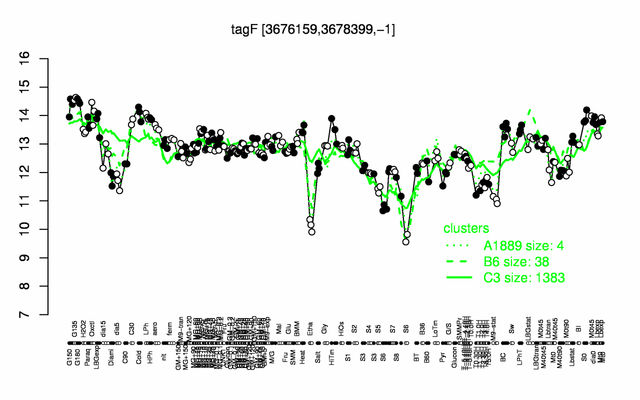
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35720
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: CDP-glycerol + (glycerophosphate)(n) = CMP + (glycerophosphate)(n+1) (according to Swiss-Prot)
- Protein family: CDP-glycerol glycerophosphotransferase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane associated PubMed
Database entries
- Structure:
- UniProt: P13485
- KEGG entry: [3]
- E.C. number: 2.7.8.12
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Edward W C Sewell, Mark P Pereira, Eric D Brown
The wall teichoic acid polymerase TagF is non-processive in vitro and amenable to study using steady state kinetic analysis.
J Biol Chem: 2009, 284(32);21132-8
[PubMed:19520862]
[WorldCat.org]
[DOI]
(P p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Alex Formstone, Rut Carballido-López, Philippe Noirot, Jeffery Errington, Dirk-Jan Scheffers
Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis.
J Bacteriol: 2008, 190(5);1812-21
[PubMed:18156271]
[WorldCat.org]
[DOI]
(I p)
Jeffrey W Schertzer, Amit P Bhavsar, Eric D Brown
Two conserved histidine residues are critical to the function of the TagF-like family of enzymes.
J Biol Chem: 2005, 280(44);36683-90
[PubMed:16141206]
[WorldCat.org]
[DOI]
(P p)
Amit P Bhavsar, Laura K Erdman, Jeffrey W Schertzer, Eric D Brown
Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid.
J Bacteriol: 2004, 186(23);7865-73
[PubMed:15547257]
[WorldCat.org]
[DOI]
(P p)
Alistair Howell, Sarah Dubrac, Kasper Krogh Andersen, David Noone, Juliette Fert, Tarek Msadek, Kevin Devine
Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach.
Mol Microbiol: 2003, 49(6);1639-55
[PubMed:12950927]
[WorldCat.org]
[DOI]
(P p)
Jeffrey W Schertzer, Eric D Brown
Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro.
J Biol Chem: 2003, 278(20);18002-7
[PubMed:12637499]
[WorldCat.org]
[DOI]
(P p)
W Liu, S Eder, F M Hulett
Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP-P.
J Bacteriol: 1998, 180(3);753-8
[PubMed:9457886]
[WorldCat.org]
[DOI]
(P p)
C Mauël, A Bauduret, C Chervet, S Beggah, D Karamata
In Bacillus subtilis 168, teichoic acid of the cross-wall may be different from that of the cylinder: a hypothesis based on transcription analysis of tag genes.
Microbiology (Reading): 1995, 141 ( Pt 10);2379-89
[PubMed:7581998]
[WorldCat.org]
[DOI]
(P p)
P M Wagner, G C Stewart
Role and expression of the Bacillus subtilis rodC operon.
J Bacteriol: 1991, 173(14);4341-6
[PubMed:1712357]
[WorldCat.org]
[DOI]
(P p)
A L Honeyman, G C Stewart
The nucleotide sequence of the rodC operon of Bacillus subtilis.
Mol Microbiol: 1989, 3(9);1257-68
[PubMed:2507871]
[WorldCat.org]
[DOI]
(P p)