HprK
| Gene name | hprK |
| Synonyms | ptsK, yvoB |
| Essential | no |
| Product | HPr kinase/ phosphorylase |
| Function | carbon catabolite repression, phosphorylation of HPr and Crh proteins at Ser46 |
| Interactions involving this protein in SubtInteract: HprK | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Protein secretion | |
| MW, pI | 34 kDa, 4.906 |
| Gene length, protein length | 930 bp, 310 aa |
| Immediate neighbours | lgt, nagA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 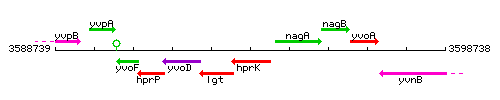
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35000
Phenotypes of a mutant
no carbon catabolite repression
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + HPr = ADP + P-Ser-HPr (according to Swiss-Prot)
- Protein family: HPrK/P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1KKM (complex of Lactobacillus casei HprK with B. subtilis HPr-Ser-P), 1KKL (complex of Lactobacillus casei HprK with B. subtilis HPr)
- UniProt: O34483
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP202 (spc), GP858 (aphA3), GP82 (cat), available in Stülke lab
- Expression vector:
- for expression/ purification from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP642, available in Stülke lab
- for expression/ purification of mutant HprK-G158A from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP650, available in Stülke lab
- for expression/ purification from E. coli with N-terminal His-tag, in pWH844: pGP205, available in Stülke lab
- for expression, purification of the N-terminal in E. coli with N-terminal His-tag, in pWH844: pGP218, available in Stülke lab
- GFP fusion:
- two-hybrid system:
- Antibody: available in Stülke lab
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Wolfgang Hillen, Erlangen University, Germany Homepage
Anne Galinier, University of Marseille, France
Your additional remarks
References
Reviews
Boris Görke, Jörg Stülke
Carbon catabolite repression in bacteria: many ways to make the most out of nutrients.
Nat Rev Microbiol: 2008, 6(8);613-24
[PubMed:18628769]
[WorldCat.org]
[DOI]
(I p)
Sandrine Poncet, Ivan Mijakovic, Sylvie Nessler, Virginie Gueguen-Chaignon, Vincent Chaptal, Anne Galinier, Grégory Boël, Alain Mazé, Josef Deutscher
HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria.
Biochim Biophys Acta: 2004, 1697(1-2);123-35
[PubMed:15023355]
[WorldCat.org]
[DOI]
(P p)
Sylvie Nessler, Sonia Fieulaine, Sandrine Poncet, Anne Galinier, Josef Deutscher, Joël Janin
HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in Gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate.
J Bacteriol: 2003, 185(14);4003-10
[PubMed:12837773]
[WorldCat.org]
[DOI]
(P p)
General Analysis, Physiology
Structural Analysis of HPrK
Enzymatic Properties, Mutation Analysis
Frédérique Pompeo, Yohann Granet, Jean-Pierre Lavergne, Christophe Grangeasse, Sylvie Nessler, Jean-Michel Jault, Anne Galinier
Regulation and mutational analysis of the HPr kinase/phosphorylase from Bacillus subtilis.
Biochemistry: 2003, 42(22);6762-71
[PubMed:12779331]
[WorldCat.org]
[DOI]
(P p)
Helena Ramström, Sarah Sanglier, Emmanuelle Leize-Wagner, Claude Philippe, Alain Van Dorsselaer, Jacques Haiech
Properties and regulation of the bifunctional enzyme HPr kinase/phosphatase in Bacillus subtilis.
J Biol Chem: 2003, 278(2);1174-85
[PubMed:12411438]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Sandrine Poncet, Anne Galinier, Vicente Monedero, Sonia Fieulaine, Joël Janin, Sylvie Nessler, José Antonio Marquez, Klaus Scheffzek, Sonja Hasenbein, Wolfgang Hengstenberg, Josef Deutscher
Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life?
Proc Natl Acad Sci U S A: 2002, 99(21);13442-7
[PubMed:12359880]
[WorldCat.org]
[DOI]
(P p)
K G Hanson, Katrin Steinhauer, Jonathan Reizer, Wolfgang Hillen, Jörg Stülke
HPr kinase/phosphatase of Bacillus subtilis: expression of the gene and effects of mutations on enzyme activity, growth and carbon catabolite repression.
Microbiology (Reading): 2002, 148(Pt 6);1805-1811
[PubMed:12055300]
[WorldCat.org]
[DOI]
(P p)
Jean-Pierre Lavergne, Jean-Michel Jault, Anne Galinier
Insights into the functioning of Bacillus subtilis HPr kinase/phosphatase: affinity for its protein substrates and role of cations and phosphate.
Biochemistry: 2002, 41(20);6218-25
[PubMed:12009882]
[WorldCat.org]
[DOI]
(P p)
Anne Galinier, Jean-Pierre Lavergne, Christophe Geourjon, Sonia Fieulaine, Sylvie Nessler, Jean-Michel Jault
A new family of phosphotransferases with a P-loop motif.
J Biol Chem: 2002, 277(13);11362-7
[PubMed:11796714]
[WorldCat.org]
[DOI]
(P p)
V Monedero, S Poncet, I Mijakovic, S Fieulaine, V Dossonnet, I Martin-Verstraete, S Nessler, J Deutscher
Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism.
EMBO J: 2001, 20(15);3928-37
[PubMed:11483496]
[WorldCat.org]
[DOI]
(P p)
J M Jault, S Fieulaine, S Nessler, P Gonzalo, A Di Pietro, J Deutscher, A Galinier
The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding.
J Biol Chem: 2000, 275(3);1773-80
[PubMed:10636874]
[WorldCat.org]
[DOI]
(P p)
HprK as a Target For Antimicrobial Compounds
Helena Ramström, Maryline Bourotte, Claude Philippe, Martine Schmitt, Jacques Haiech, Jean-Jacques Bourguignon
Heterocyclic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/phosphatase from Bacillus subtilis.
J Med Chem: 2004, 47(9);2264-75
[PubMed:15084125]
[WorldCat.org]
[DOI]
(P p)