Difference between revisions of "ClpP"
(→Biological materials) |
(→Biological materials) |
||
| Line 100: | Line 100: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' ''clpP::spec'' and ''clpP::cat'' available from the [[Hamoen]] Lab [http://subtiwiki.uni-goettingen.de/wiki/index.php/Leendert_Hamoen Subtiwiki] |
* '''Expression vector:''' | * '''Expression vector:''' | ||
Revision as of 12:29, 28 April 2009
- Description: ATP-dependent Clp protease proteolytic subunit (class III heat-shock protein)
| Gene name | clpP |
| Synonyms | yvdN |
| Essential | no |
| Product | ATP-dependent Clp protease proteolytic subunit |
| Function | protein degradation |
| MW, pI | 21 kDa, 5.008 |
| Gene length, protein length | 591 bp, 197 aa |
| Immediate neighbours | trnQ-Arg, pgcM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 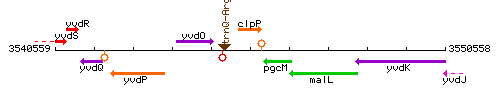
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (both single copy and 2th copy in amyE locus, also as CFP and YFP variants) available from the Hamoen Lab Subtiwiki
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
- Petersohn et al. (2001) Global Analysis of the General Stress Response of Bacillus subtilis. J Bacteriol. 183: 5617-5631 PubMed
- Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. PubMed
- Gerth, U., Krüger, E., Derré, I., Msadek, T. and Hecker, M. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the ClpP protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28: 787-802. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed