Difference between revisions of "Aag"
| Line 41: | Line 41: | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/yxlJ.html] | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/yxlJ-yxzF.html] |
* '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG12555] | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG12555] | ||
Revision as of 14:42, 10 March 2009
- Description: 3-methyladenine DNA glycosylase
| Gene name | aag |
| Synonyms | yxlJ |
| Essential | no |
| Product | 3-methyladenine DNA glycosylase |
| Function | DNA repair |
| MW, pI | 21 kDa, 6.117 |
| Gene length, protein length | 588 bp, 196 aa |
| Immediate neighbours | yxzF, katX |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 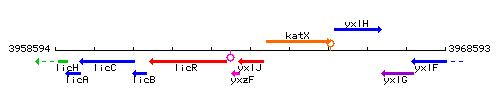
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: removes hypoxanthine, 3-alkylated purines, and 1,N6-ethenoadenine from DNA; hypoxanthine and 1,N6-ethenoadenine are the preferred substrates PubMed
- Protein family: AAG family of 3-methyladenine DNA glycosylases
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry:
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Aamodt et al. (2004) The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates. J. Biol. Chem. 279: 13601-13606-page. PubMed