Difference between revisions of "Hbs"
(→Biological materials) |
(→Original publications) |
||
| Line 157: | Line 157: | ||
<pubmed>11931565,</pubmed> | <pubmed>11931565,</pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>1382620, 1902464,10715001,9068655,9894920,16267290, 19458035, 19210617, 18763711, 17218307 21954439 22517742 23033921, 10231583, </pubmed> | + | <pubmed>1382620, 1902464,10715001,9068655,9894920,16267290, 19458035, 19210617, 18763711, 17218307 21954439 22517742 23033921, 10231583, 9106208</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:10, 10 August 2015
- Description: non-specific DNA-binding protein Hbsu
| Gene name | hbs |
| Synonyms | dbpA, hupA |
| Essential | yes PubMed |
| Product | non-specific DNA-binding protein Hbsu |
| Function | DNA packaging, function of the signal recognition complex |
| Gene expression levels in SubtiExpress: hbs | |
| MW, pI | 9 kDa, 9.501 |
| Gene length, protein length | 276 bp, 92 aa |
| Immediate neighbours | folE, spoIVA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed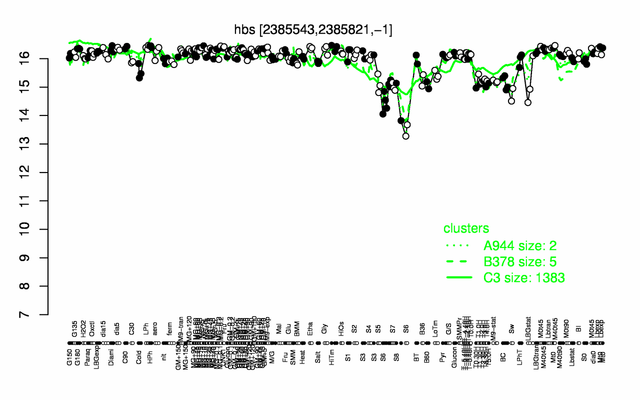
| |
Contents
Categories containing this gene/protein
DNA condensation/ segregation, essential genes, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22790
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU22790
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: DbpA subfamily (according to Swiss-Prot)
- Paralogous protein(s): YonN
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU22790
- Structure: 1HUU (Geobacillus stearothermophilus)
- UniProt: P08821
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: hbs PubMed
- Regulation:
- Additional information:
- mRNA is protected from degradation by RnjA by initiating ribosomes PubMed
- Hbs is present with about 50,000 copies per genome in vegetative cells and spores, it is one of the most abundant proteins in B. subtilis PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 19917 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 41521 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Fabian Commichau's lab
- Antibody:
Labs working on this gene/protein
- Ciaran Condon, IBPC, Paris, France Homepage
- Mohamed Marahiel, University of Marburg, Germany Homepage
Your additional remarks
References
Reviews
Original publications
Imke G de Jong, Jan-Willem Veening, Oscar P Kuipers
Single cell analysis of gene expression patterns during carbon starvation in Bacillus subtilis reveals large phenotypic variation.
Environ Microbiol: 2012, 14(12);3110-21
[PubMed:23033921]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Christopher Collier, Cristina Machón, Geoff S Briggs, Wiep Klaas Smits, Panos Soultanas
Untwisting of the DNA helix stimulates the endonuclease activity of Bacillus subtilis Nth at AP sites.
Nucleic Acids Res: 2012, 40(2);739-50
[PubMed:21954439]
[WorldCat.org]
[DOI]
(I p)
Roula Daou-Chabo, Ciarán Condon
RNase J1 endonuclease activity as a probe of RNA secondary structure.
RNA: 2009, 15(7);1417-25
[PubMed:19458035]
[WorldCat.org]
[DOI]
(I p)
Roula Daou-Chabo, Nathalie Mathy, Lionel Bénard, Ciarán Condon
Ribosomes initiating translation of the hbs mRNA protect it from 5'-to-3' exoribonucleolytic degradation by RNase J1.
Mol Microbiol: 2009, 71(6);1538-50
[PubMed:19210617]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Nora Au, Elke Kuester-Schoeck, Veena Mandava, Laura E Bothwell, Susan P Canny, Karen Chachu, Sierra A Colavito, Shakierah N Fuller, Eli S Groban, Laura A Hensley, Theresa C O'Brien, Amish Shah, Jessica T Tierney, Louise L Tomm, Thomas M O'Gara, Alexi I Goranov, Alan D Grossman, Charles M Lovett
Genetic composition of the Bacillus subtilis SOS system.
J Bacteriol: 2005, 187(22);7655-66
[PubMed:16267290]
[WorldCat.org]
[DOI]
(P p)
M A Ross, P Setlow
The Bacillus subtilis HBsu protein modifies the effects of alpha/beta-type, small acid-soluble spore proteins on DNA.
J Bacteriol: 2000, 182(7);1942-8
[PubMed:10715001]
[WorldCat.org]
[DOI]
(P p)
S Fernández, J C Alonso
Bacillus subtilis sequence-independent DNA-binding and DNA-bending protein Hbsu negatively controls its own synthesis.
Gene: 1999, 231(1-2);187-93
[PubMed:10231583]
[WorldCat.org]
[DOI]
(P p)
P Köhler, M A Marahiel
Mutational analysis of the nucleoid-associated protein HBsu of Bacillus subtilis.
Mol Gen Genet: 1998, 260(5);487-91
[PubMed:9894920]
[WorldCat.org]
[DOI]
(P p)
S Fernández, F Rojo, J C Alonso
The Bacillus subtilis chromatin-associated protein Hbsu is involved in DNA repair and recombination.
Mol Microbiol: 1997, 23(6);1169-79
[PubMed:9106208]
[WorldCat.org]
[DOI]
(P p)
P Köhler, M A Marahiel
Association of the histone-like protein HBsu with the nucleoid of Bacillus subtilis.
J Bacteriol: 1997, 179(6);2060-4
[PubMed:9068655]
[WorldCat.org]
[DOI]
(P p)
B Micka, M A Marahiel
The DNA-binding protein HBsu is essential for normal growth and development in Bacillus subtilis.
Biochimie: 1992, 74(7-8);641-50
[PubMed:1382620]
[WorldCat.org]
[DOI]
(P p)
B Micka, N Groch, U Heinemann, M A Marahiel
Molecular cloning, nucleotide sequence, and characterization of the Bacillus subtilis gene encoding the DNA-binding protein HBsu.
J Bacteriol: 1991, 173(10);3191-8
[PubMed:1902464]
[WorldCat.org]
[DOI]
(P p)