Difference between revisions of "KtrC"
| Line 39: | Line 39: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 70: | Line 66: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 90: | Line 83: | ||
* '''[[Domains]]:''' | * '''[[Domains]]:''' | ||
** contains a [[RCK_N domain]] at the N-terminus (according to UniProt, [http://www.uniprot.org/uniprot/?query=domain:%22RCK+N-terminal+domain*%22]) | ** contains a [[RCK_N domain]] at the N-terminus (according to UniProt, [http://www.uniprot.org/uniprot/?query=domain:%22RCK+N-terminal+domain*%22]) | ||
| − | ** contains a [[RCK_C domain]] at the C-terminus (according to UniProt, [http://www.uniprot.org/uniprot/?query=domain:%22RCK+C-terminal+domain*%22]) | + | ** contains a c-di-AMP-binding [[RCK_C domain]] at the C-terminus (according to UniProt, [http://www.uniprot.org/uniprot/?query=domain:%22RCK+C-terminal+domain*%22]) {{PubMed|23671116}} |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| + | ** the protein binds c-di-AMP {{PubMed|23671116}} | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| Line 155: | Line 149: | ||
=References= | =References= | ||
| − | <pubmed>12562800,, </pubmed> | + | <pubmed>12562800,23671116, </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:17, 1 December 2014
| Gene name | ktrC |
| Synonyms | ylxV, yzaC, ykqB |
| Essential | no |
| Product | low affinity potassium transporter KtrC-KtrD, peripheric membrane component (proton symport) |
| Function | potassium uptake |
| Gene expression levels in SubtiExpress: ktrC | |
| Interactions involving this protein in SubtInteract: KtrC | |
| Metabolic function and regulation of this protein in SubtiPathways: Metal ion homeostasis, ktrC | |
| MW, pI | 24 kDa, 5.63 |
| Gene length, protein length | 663 bp, 221 aa |
| Immediate neighbours | ykqA, adeC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 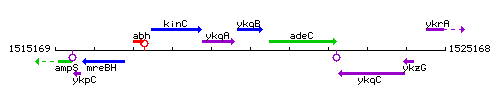
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transporters/ other, metal ion homeostasis (K, Na, Ca, Mg), coping with hyper-osmotic stress, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14510
Phenotypes of a mutant
Database entries
- BsubCyc: BSU14510
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s): KtrA
Extended information on the protein
- Kinetic information:
- Domains:
- contains a RCK_N domain at the N-terminus (according to UniProt, [2])
- contains a c-di-AMP-binding RCK_C domain at the C-terminus (according to UniProt, [3]) PubMed
- Modification:
- Effectors of protein activity:
- the protein binds c-di-AMP PubMed
- Localization: peripheral membrane protein PubMed
Database entries
- BsubCyc: BSU14510
- Structure:
- UniProt: P39760
- KEGG entry: [4]
- E.C. number:
Additional information
Expression and regulation
- Operon: ktrC PubMed
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 576 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 453 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 601 PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Erhard Bremer, University of Marburg, Germany homepage
Your additional remarks
References
Rebecca M Corrigan, Ivan Campeotto, Tharshika Jeganathan, Kevin G Roelofs, Vincent T Lee, Angelika Gründling
Systematic identification of conserved bacterial c-di-AMP receptor proteins.
Proc Natl Acad Sci U S A: 2013, 110(22);9084-9
[PubMed:23671116]
[WorldCat.org]
[DOI]
(I p)
Gudrun Holtmann, Evert P Bakker, Nobuyuki Uozumi, Erhard Bremer
KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity.
J Bacteriol: 2003, 185(4);1289-98
[PubMed:12562800]
[WorldCat.org]
[DOI]
(P p)