Difference between revisions of "AhpF"
m (Reverted edits by 134.76.70.252 (talk) to last revision by 134.76.38.147) |
|||
| Line 127: | Line 127: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 5837 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 4363 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 5017 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 13:53, 17 April 2014
- Description: alkyl hydroperoxide reductase (large subunit) / NADH dehydrogenase
| Gene name | ahpF |
| Synonyms | ndh |
| Essential | no |
| Product | alkyl hydroperoxide reductase (large subunit) / NADH dehydrogenase |
| Function | resistance against peroxide stres |
| Gene expression levels in SubtiExpress: ahpF | |
| Metabolic function and regulation of this protein in SubtiPathways: ahpF | |
| MW, pI | 54 kDa, 4.705 |
| Gene length, protein length | 1527 bp, 509 aa |
| Immediate neighbours | ahpC, bglA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 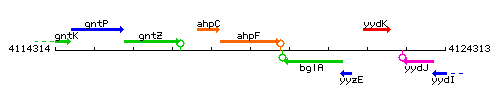
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU40100
Phenotypes of a mutant
Database entries
- BsubCyc: BSU40100
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: NADH + acceptor = NAD+ + reduced acceptor (according to Swiss-Prot)
- Protein family: class-II pyridine nucleotide-disulfide oxidoreductase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU40100
- UniProt: P42974
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 5837 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 4363 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 5017 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
A Sakai, K Katayama, T Katsuragi, Y Tani
Glycolaldehyde-forming route in Bacillus subtilis in relation to vitamin B6 biosynthesis.
J Biosci Bioeng: 2001, 91(2);147-52
[PubMed:16232966]
[WorldCat.org]
[DOI]
(P p)
A F Herbig, J D Helmann
Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA.
Mol Microbiol: 2001, 41(4);849-59
[PubMed:11532148]
[WorldCat.org]
[DOI]
(P p)
N Bsat, L Chen, J D Helmann
Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes.
J Bacteriol: 1996, 178(22);6579-86
[PubMed:8932315]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, S Engelmann, R Schmid, M Hecker
General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon.
J Bacteriol: 1996, 178(22);6571-8
[PubMed:8932314]
[WorldCat.org]
[DOI]
(P p)