Difference between revisions of "Fur"
| Line 64: | Line 64: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU23520&redirect=T BSU23520] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/fur.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/fur.html] | ||
| Line 101: | Line 102: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU23520&redirect=T BSU23520] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 14:08, 2 April 2014
- Description: transcription regulator of iron homoeostasis
| Gene name | fur |
| Synonyms | yqkL |
| Essential | no |
| Product | transcriptional repressor Fur family |
| Function | regulation of iron homoeostasis
and transcription of ferri-siderophore uptake genes |
| Gene expression levels in SubtiExpress: fur
and transcription of ferri-siderophore uptake genes | |
| Metabolic function and regulation of this protein in SubtiPathways: Metal ion homeostasis, fur | |
| MW, pI | 17 kDa, 5.374 |
| Gene length, protein length | 447 bp, 149 aa |
| Immediate neighbours | yqzK, spoIIM |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 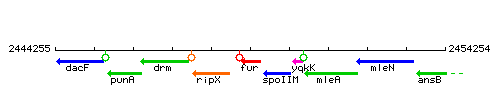
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
iron metabolism, transcription factors and their control
- see also: glutamate metabolism
This gene is a member of the following regulons
The Fur regulon
The gene
Basic information
- Locus tag: BSU23520
Phenotypes of a mutant
- no growth with glucose and ammonium as single sources of carbon and nitrogen, respectively (due to FsrA-mediated repression of the gltA-gltB operon) PubMed
- poor frowth on lactate as single carbon source (due to overexpression of FsrA-mediated repression of the lutA-lutB-lutC operon, can be suppressed by inactivation of fsrA or fbpB) PubMed
- transcription profile of a fur mutant strain: GEO PubMed
Database entries
- BsubCyc: BSU23520
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Fur family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): contains an iron-sulfur cluster
- Effectors of protein activity:
- DNA binding activity (repression) is triggered by binding of Fe(II) PubMed
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU23520
- Structure:
- UniProt: P54574
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: fur PubMed
- Additional information:
Biological materials
- Mutant: HB6543 (aphA3), available in the John Helmann lab; also available in the Stülke lab GP879 (fur::mls) and GP868 (fur::mls, perR::spc)
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Reviews
The Fur regulon
Addititonal publications: PubMed
Other original publications
Addititonal publications: PubMed
Gregory T Smaldone, Haike Antelmann, Ahmed Gaballa, John D Helmann
The FsrA sRNA and FbpB protein mediate the iron-dependent induction of the Bacillus subtilis lutABC iron-sulfur-containing oxidases.
J Bacteriol: 2012, 194(10);2586-93
[PubMed:22427629]
[WorldCat.org]
[DOI]
(I p)
David P Giedroc
Hydrogen peroxide sensing in Bacillus subtilis: it is all about the (metallo)regulator.
Mol Microbiol: 2009, 73(1);1-4
[PubMed:19508286]
[WorldCat.org]
[DOI]
(I p)
Ahmed Gaballa, Haike Antelmann, Claudio Aguilar, Sukhjit K Khakh, Kyung-Bok Song, Gregory T Smaldone, John D Helmann
The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins.
Proc Natl Acad Sci U S A: 2008, 105(33);11927-32
[PubMed:18697947]
[WorldCat.org]
[DOI]
(I p)
Falko Hochgräfe, Carmen Wolf, Stephan Fuchs, Manuel Liebeke, Michael Lalk, Susanne Engelmann, Michael Hecker
Nitric oxide stress induces different responses but mediates comparable protein thiol protection in Bacillus subtilis and Staphylococcus aureus.
J Bacteriol: 2008, 190(14);4997-5008
[PubMed:18487332]
[WorldCat.org]
[DOI]
(I p)
Ahmed Gaballa, John D Helmann
Substrate induction of siderophore transport in Bacillus subtilis mediated by a novel one-component regulator.
Mol Microbiol: 2007, 66(1);164-73
[PubMed:17725565]
[WorldCat.org]
[DOI]
(P p)
Marcus Miethke, Helga Westers, Evert-Jan Blom, Oscar P Kuipers, Mohamed A Marahiel
Iron starvation triggers the stringent response and induces amino acid biosynthesis for bacillibactin production in Bacillus subtilis.
J Bacteriol: 2006, 188(24);8655-7
[PubMed:17012385]
[WorldCat.org]
[DOI]
(P p)
Juliane Ollinger, Kyung-Bok Song, Haike Antelmann, Michael Hecker, John D Helmann
Role of the Fur regulon in iron transport in Bacillus subtilis.
J Bacteriol: 2006, 188(10);3664-73
[PubMed:16672620]
[WorldCat.org]
[DOI]
(P p)
Mayuree Fuangthong, John D Helmann
Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis.
J Bacteriol: 2003, 185(21);6348-57
[PubMed:14563870]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, Charles M Moore, Qiang Que, Tao Wang, Rick W Ye, John D Helmann
The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons.
Mol Microbiol: 2003, 49(6);1477-91
[PubMed:12950915]
[WorldCat.org]
[DOI]
(P p)
Min Cao, Tao Wang, Rick Ye, John D Helmann
Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis sigma(W) and sigma(M) regulons.
Mol Microbiol: 2002, 45(5);1267-76
[PubMed:12207695]
[WorldCat.org]
[DOI]
(P p)
Mayuree Fuangthong, Andrew F Herbig, Nada Bsat, John D Helmann
Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible.
J Bacteriol: 2002, 184(12);3276-86
[PubMed:12029044]
[WorldCat.org]
[DOI]
(P p)
Tamara Hoffmann, Alexandra Schütz, Margot Brosius, Andrea Völker, Uwe Völker, Erhard Bremer
High-salinity-induced iron limitation in Bacillus subtilis.
J Bacteriol: 2002, 184(3);718-27
[PubMed:11790741]
[WorldCat.org]
[DOI]
(P p)
N Bsat, J D Helmann
Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo.
J Bacteriol: 1999, 181(14);4299-307
[PubMed:10400588]
[WorldCat.org]
[DOI]
(P p)
N Bsat, A Herbig, L Casillas-Martinez, P Setlow, J D Helmann
Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors.
Mol Microbiol: 1998, 29(1);189-98
[PubMed:9701813]
[WorldCat.org]
[DOI]
(P p)