Difference between revisions of "MtnD"
| Line 37: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| − | {{SubtiWiki category|[[biosynthesis/ acquisition of amino acids]]}} | + | {{SubtiWiki category|[[biosynthesis/ acquisition of amino acids]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 64: | Line 61: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 82: | Line 76: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 112: | Line 106: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=mtnD_1429584_1430120_1 mtnD] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=mtnD_1429584_1430120_1 mtnD] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/pubmed/12022921 PubMed] | + | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/pubmed/12022921 PubMed] |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 121: | Line 115: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 142: | Line 137: | ||
=References= | =References= | ||
| − | <pubmed>12022921,11914366,,12107147, 15102328 12787499 18039762 </pubmed> | + | <pubmed>12022921,11914366,,12107147, 15102328 12787499 18039762 15378759</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:33, 5 March 2014
- Description: 1,2,-dihydroxy-3-keto-5-methylthiopentene dioxygenase
| Gene name | mtnD |
| Synonyms | ykrZ |
| Essential | no |
| Product | 1,2,-dihydroxy-3-keto-5-methylthiopentene dioxygenase |
| Function | methionine salvage |
| Gene expression levels in SubtiExpress: mtnD | |
| Metabolic function and regulation of this protein in SubtiPathways: mtnD | |
| MW, pI | 20 kDa, 4.408 |
| Gene length, protein length | 534 bp, 178 aa |
| Immediate neighbours | mtnB, ykvA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 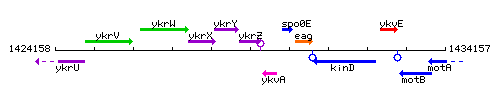
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed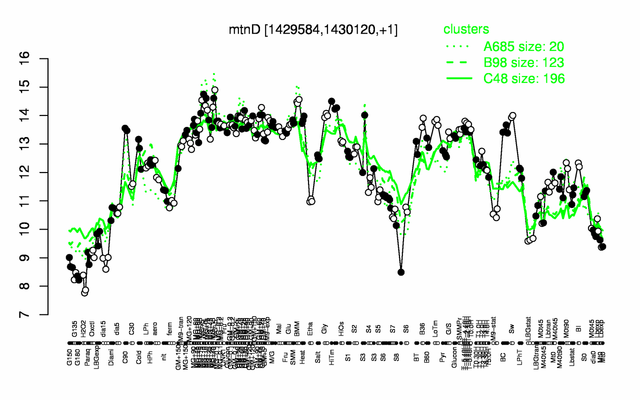
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13620
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 1,2-dihydroxy-5-(methylthio)pent-1-en-3-one + O2 = 3-(methylthio)propanoate + formate + CO (according to Swiss-Prot)
- Protein family: acireductone dioxygenase (ARD) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O31669
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism: S-box: transcription termination/ antitermination, the S-box riboswitch binds S-adenosylmethionine resulting in termination PubMed
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References