Difference between revisions of "FtsA"
(→Phenotypes of a mutant) |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' cell | + | * '''Description:''' [[cell division]] protein, membrane anchor for [[FtsZ]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || cell | + | |style="background:#ABCDEF;" align="center"| '''Product''' || [[cell division]] protein |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || formation of Z-ring | |style="background:#ABCDEF;" align="center"|'''Function''' || formation of Z-ring | ||
| Line 87: | Line 87: | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * '''Cofactors:''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 93: | Line 93: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
** [[FtsZ]]-[[FtsA]] {{PubMed|16159787}} | ** [[FtsZ]]-[[FtsA]] {{PubMed|16159787}} | ||
| + | ** [[PlsX]]-[[FtsA]] {{PubMed|24224907}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 148: | Line 149: | ||
<pubmed> 19680248 </pubmed> | <pubmed> 19680248 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>16796675,12081956,16159787,11298280,18776011,9287012,1569582,10878122,7592498, 20566861 23036588 23701187 24218584</pubmed> | + | <pubmed>16796675,12081956,16159787,11298280,18776011,9287012,1569582,10878122,7592498, 20566861 23036588 23701187 24218584 24224907</pubmed> |
| − | + | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 19:22, 12 January 2014
- Description: cell division protein, membrane anchor for FtsZ
| Gene name | ftsA |
| Synonyms | spoIIN |
| Essential | no |
| Product | cell division protein |
| Function | formation of Z-ring |
| Gene expression levels in SubtiExpress: ftsA | |
| Interactions involving this protein in SubtInteract: FtsA | |
| MW, pI | 47 kDa, 5.094 |
| Gene length, protein length | 1320 bp, 440 aa |
| Immediate neighbours | sbp, ftsZ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 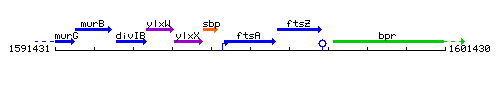
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell division, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15280
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsA/mreB family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P28264
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Original Publications