Difference between revisions of "FosB"
| Line 22: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[yndM]]'', ''[[lexA]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[yndM]]'', ''[[lexA]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU17840 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU17840 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU17840 | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU17840 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU17840 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU17840 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:yndN_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:yndN_context.gif]] | ||
Revision as of 10:20, 14 May 2013
- Description: bacillithiol-S-transferase; confers resistence against antimicrobial compounds from B. amyloliquefaciens
| Gene name | fosB |
| Synonyms | yndN |
| Essential | no |
| Product | fosfomycin resistance protein |
| Function | confers resistence against antimicrobial compounds from B. amyloliquefaciens |
| Gene expression levels in SubtiExpress: fosB | |
| MW, pI | 17 kDa, 6.137 |
| Gene length, protein length | 432 bp, 144 aa |
| Immediate neighbours | yndM, lexA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 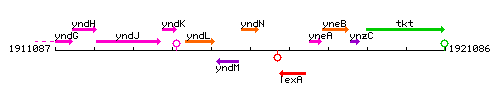
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell envelope stress proteins (controlled by SigM, V, W, X, Y), resistance against toxins/ antibiotics
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17840
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: FosB subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O31817
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: fosB PubMed
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Alexandra A Roberts, Sunil V Sharma, Andrew W Strankman, Shayla R Duran, Mamta Rawat, Chris J Hamilton
Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus.
Biochem J: 2013, 451(1);69-79
[PubMed:23256780]
[WorldCat.org]
[DOI]
(I p)
Alexander P Lamers, Mary E Keithly, Kwangho Kim, Paul D Cook, Donald F Stec, Kelly M Hines, Gary A Sulikowski, Richard N Armstrong
Synthesis of bacillithiol and the catalytic selectivity of FosB-type fosfomycin resistance proteins.
Org Lett: 2012, 14(20);5207-9
[PubMed:23030527]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Bronwyn G Butcher, John D Helmann
Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli.
Mol Microbiol: 2006, 60(3);765-82
[PubMed:16629676]
[WorldCat.org]
[DOI]
(P p)
M Cao, B A Bernat, Z Wang, R N Armstrong, J D Helmann
FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma(W), an extracytoplasmic-function sigma factor in Bacillus subtilis.
J Bacteriol: 2001, 183(7);2380-3
[PubMed:11244082]
[WorldCat.org]
[DOI]
(P p)
23256780