Difference between revisions of "FloT"
(→References) |
|||
| Line 58: | Line 58: | ||
** delayed onset of sporulation, reduced sporulation frequency | ** delayed onset of sporulation, reduced sporulation frequency | ||
** defect in motility {{PubMed|22753055}} | ** defect in motility {{PubMed|22753055}} | ||
| + | ** reduced [[protein secretion]] {{PubMed|23651456}} | ||
** a ''[[floT]] [[floA]]'' double mutant does not induce [[KinC]]-dependent biofilm formation upon addition of surfactin {{PubMed|20713508}} | ** a ''[[floT]] [[floA]]'' double mutant does not induce [[KinC]]-dependent biofilm formation upon addition of surfactin {{PubMed|20713508}} | ||
** a ''[[floT]] [[floA]]'' double mutant has a strong synthetic defect in motility, cell morphology, and transformation efficiency {{PubMed|22753055}} | ** a ''[[floT]] [[floA]]'' double mutant has a strong synthetic defect in motility, cell morphology, and transformation efficiency {{PubMed|22753055}} | ||
| Line 95: | Line 96: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| + | ** forms homo-oligomers {{PubMed|23651456}} | ||
** [[YuaF]]-[[FloT]] {{PubMed|22753055}} | ** [[YuaF]]-[[FloT]] {{PubMed|22753055}} | ||
** [[FloT]]-[[FtsH]] {{PubMed|22882210}} | ** [[FloT]]-[[FtsH]] {{PubMed|22882210}} | ||
| + | ** [[FloT]]-[[FloA]] {{PubMed|23651456}} | ||
| + | ** [[FloT]]-[[SecY]] {{PubMed|23651456}} | ||
| + | ** [[FloT]]-[[FtsX]] {{PubMed|23651456}} | ||
| + | ** [[FloT]]-[[OppA]] {{PubMed|23651456}} | ||
| + | ** [[FloT]]-[[SdhA]] {{PubMed|23651456}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
** membrane associated [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | ** membrane associated [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | ||
** co-localizes with [[FloA]] and [[KinC]] in discrete foci membrane {{PubMed|20713508}} | ** co-localizes with [[FloA]] and [[KinC]] in discrete foci membrane {{PubMed|20713508}} | ||
| − | ** forms focal | + | ** forms discrete focal structures in the cytoplasma membrane {{PubMed|22753055}} |
=== Database entries === | === Database entries === | ||
| Line 149: | Line 156: | ||
=References= | =References= | ||
| − | + | ||
| − | <pubmed>9987136,22753055, 12107147, 18763711, 19383680 20713508 | + | <pubmed>9987136,22753055, 12107147, 18763711, 19383680 20713508 23651456 22178969 ,23249255 22882210</pubmed> |
| − | |||
| − | |||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:39, 10 May 2013
- Description: similar to flotillin 1, orchestration of physiological processes in lipid microdomains, involved in the control of membrane fluidity, confers (together with YuaF) resistance to cefuroxime
| Gene name | floT |
| Synonyms | yuaG, yuaH |
| Essential | no |
| Product | bacterial flotillin-like protein |
| Function | involved in the control of membrane fluidity |
| Gene expression levels in SubtiExpress: floT | |
| Interactions involving this protein in SubtInteract: FloT | |
| MW, pI | 55 kDa, 5.135 |
| Gene length, protein length | 1527 bp, 509 aa |
| Immediate neighbours | yuaI, yuaF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 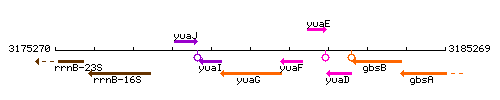
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biofilm formation, membrane dynamics, membrane proteins sporulation/ other, cell envelope stress proteins (controlled by SigM, V, W, X, Y)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31010
Phenotypes of a mutant
- delayed onset of sporulation, reduced sporulation frequency
- defect in motility PubMed
- reduced protein secretion PubMed
- a floT floA double mutant does not induce KinC-dependent biofilm formation upon addition of surfactin PubMed
- a floT floA double mutant has a strong synthetic defect in motility, cell morphology, and transformation efficiency PubMed
- a floT floA double mutant has a sporulation defect, due to the lack of FtsH PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O32076
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References