Difference between revisions of "DacC"
| Line 35: | Line 35: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 109: | Line 105: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dacC_1998340_1999815_-1 dacC] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dacC_1998340_1999815_-1 dacC] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigH]] {{PubMed|9733705}}, [[SigB]] {{PubMed|10482513}} | + | * '''[[Sigma factor]]:''' [[SigH]] {{PubMed|9733705}}, [[SigB]] {{PubMed|10482513}} |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 136: | Line 132: | ||
=References= | =References= | ||
| − | <pubmed> 9733705 10482513 19758330 14731276,17582436 ,9864321, 21821766 22029692 </pubmed> | + | <pubmed> 9733705 10482513 19758330 14731276,17582436 ,9864321, 21821766 22029692 23560856 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:29, 13 April 2013
- Description: penicillin-binding protein 4A, D-alanyl-D-alanine carboxypeptidase
| Gene name | dacC |
| Synonyms | pbp |
| Essential | no |
| Product | penicillin-binding protein 4A, D-alanyl-D-alanine carboxypeptidase |
| Function | carboxypeptidase |
| Gene expression levels in SubtiExpress: dacC | |
| MW, pI | 52 kDa, 5.413 |
| Gene length, protein length | 1473 bp, 491 aa |
| Immediate neighbours | ppsA, galM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 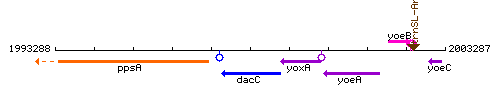
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed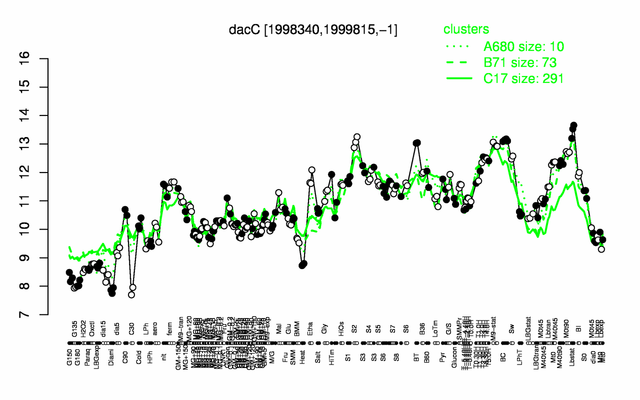
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU18350
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relexed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: cleavage ofD-Ala-D-Ala interpeptide bridges in peptidoglycan PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- may be part of the cell wall biosynthetic complex PubMed
Database entries
- UniProt: P39844
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References