Difference between revisions of "TufA"
| Line 90: | Line 90: | ||
* '''Modification:''' phosphorylated on Thr-384 by [[PrkC]], dephosphorylated by [[PrpC]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19246764 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | * '''Modification:''' phosphorylated on Thr-384 by [[PrkC]], dephosphorylated by [[PrpC]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19246764 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ||
| + | ** Cys83 is S-bacillithiolated in B. subtilis and other Bacillus species [http://www.ncbi.nlm.nih.gov/pubmed/22938038 PubMed] | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 153: | Line 154: | ||
=References= | =References= | ||
| − | <pubmed>19246764,20133608 , 19246764, 17726680, 18763711 </pubmed> | + | <pubmed>22938038,19246764,20133608 , 19246764, 17726680, 18763711 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:18, 6 September 2012
- Description: elongation factor Tu
| Gene name | tufA |
| Synonyms | |
| Essential | yes PubMed |
| Product | elongation factor Tu |
| Function | translation |
| Gene expression levels in SubtiExpress: tufA | |
| Interactions involving this protein in SubtInteract: TufA | |
| MW, pI | 43 kDa, 4.723 |
| Gene length, protein length | 1188 bp, 396 aa |
| Immediate neighbours | fusA, ybaC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 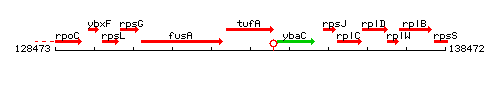
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed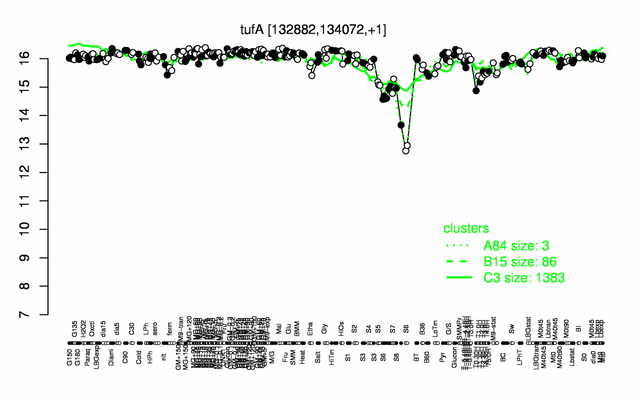
| |
Contents
Categories containing this gene/protein
translation, essential genes, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01130
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: EF-Tu/EF-1A subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated on Thr-384 by PrkC, dephosphorylated by PrpC PubMed, PubMed
- Cys83 is S-bacillithiolated in B. subtilis and other Bacillus species PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P33166
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References