Difference between revisions of "GuaB"
(→Extended information on the protein) |
(→Extended information on the protein) |
||
| Line 87: | Line 87: | ||
* '''Modification:''' | * '''Modification:''' | ||
** phosphorylated (STY) [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ** phosphorylated (STY) [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ||
| − | ** S- | + | ** Cys308 is S-cysteinylated after diamide stress () [http://www.ncbi.nlm.nih.gov/pubmed/17611193 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] |
| − | ** S-bacillithiolated by NaOCl stress | + | ** Cys308 is S-bacillithiolated by NaOCl stress in B. subtilis and other Bacillus species [http://www.ncbi.nlm.nih.gov/pubmed/22938038 PubMed] |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
Revision as of 08:10, 6 September 2012
- Description: IMP dehydrogenase
| Gene name | guaB |
| Synonyms | guaA |
| Essential | yes PubMed |
| Product | IMP dehydrogenase |
| Function | biosynthesis of GMP |
| Gene expression levels in SubtiExpress: GuaB | |
| Metabolic function and regulation of this protein in SubtiPathways: Purine synthesis, Nucleotides (regulation) | |
| MW, pI | 52 kDa, 6.168 |
| Gene length, protein length | 1464 bp, 488 aa |
| Immediate neighbours | yaaC, dacA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 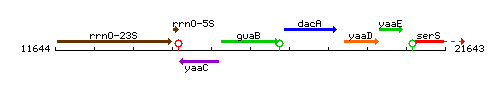
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed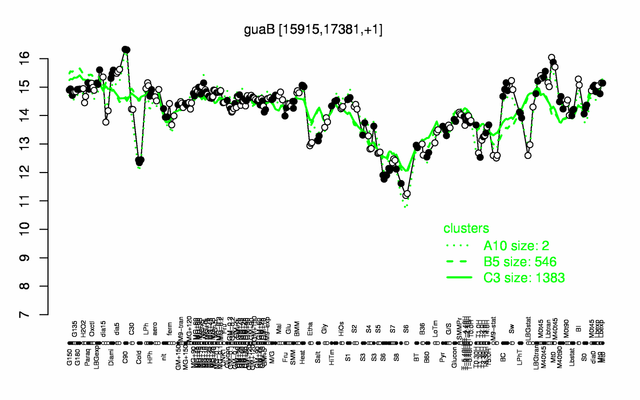
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of nucleotides, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00090
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Inosine 5'-phosphate + NAD+ + H2O = xanthosine 5'-phosphate + NADH (according to Swiss-Prot)
- Protein family: IMPDH/GMPR family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- inhibition of enzymatic activity by (p)ppGpp during the ´stringent response´PubMed
Database entries
- Structure: 1VRD (from Thermotoga maritima msb8, 60% identity, 80% similarity)
- UniProt: P21879
- KEGG entry: [3]
- E.C. number: 1.1.1.205
Additional information
Expression and regulation
- Operon: guaB PubMed
- Sigma factor:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Christine Eymann, Dörte Becher, Jörg Bernhardt, Katrin Gronau, Anja Klutzny, Michael Hecker
Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis.
Proteomics: 2007, 7(19);3509-26
[PubMed:17726680]
[WorldCat.org]
[DOI]
(P p)
Falko Hochgräfe, Jörg Mostertz, Dierk-Christoph Pöther, Dörte Becher, John D Helmann, Michael Hecker
S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress.
J Biol Chem: 2007, 282(36);25981-5
[PubMed:17611193]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Yoshiko Nakaura, Robert P Shivers, Hirotake Yamaguchi, Richard Losick, Yasutaro Fujita, Abraham L Sonenshein
Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis.
J Bacteriol: 2003, 185(6);1911-22
[PubMed:12618455]
[WorldCat.org]
[DOI]
(P p)
H H Saxild, P Nygaard
Regulation of levels of purine biosynthetic enzymes in Bacillus subtilis: effects of changing purine nucleotide pools.
J Gen Microbiol: 1991, 137(10);2387-94
[PubMed:1722815]
[WorldCat.org]
[DOI]
(P p)
J M Lopez, A Dromerick, E Freese
Response of guanosine 5'-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation.
J Bacteriol: 1981, 146(2);605-13
[PubMed:6111556]
[WorldCat.org]
[DOI]
(P p)