Difference between revisions of "GlcT"
(→Original publications) |
|||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || control of glucose uptake | |style="background:#ABCDEF;" align="center"|'''Function''' || control of glucose uptake | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU13880 glcT] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/GlcT GlcT] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/GlcT GlcT] | ||
Revision as of 08:59, 7 August 2012
- Description: transcriptional antiterminator , controls expression of the ptsG-ptsH-ptsI operon
| Gene name | glcT |
| Synonyms | ykwA |
| Essential | no |
| Product | transcriptional antiterminator of the ptsG-ptsH-ptsI operon |
| Function | control of glucose uptake |
| Gene expression levels in SubtiExpress: glcT | |
| Interactions involving this protein in SubtInteract: GlcT | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 33,0 kDa, 7.01 |
| Gene length, protein length | 855 bp, 285 amino acids |
| Immediate neighbours | ykvZ, ptsG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 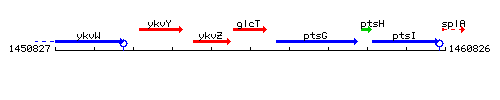
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, transcription factors and their control, RNA binding regulators, phosphoproteins
This gene is a member of the following regulons
The GlcT regulon: ptsG-ptsH-ptsI
The gene
Basic information
- Locus tag: BSU13880
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription antiterminator , RNA-binding protein, binds the ptsG RAT sequence
- Protein family: transcription antiterminator of the BglG/ SacY family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation (His104)
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O31691
- KEGG entry: [2]
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: available in Stülke lab:
- Expression vector:
- pGP124 (full length, in pWH844), available in Stülke lab
- pGP114 (amino acids 1-60, RNA-binding domain, in pWH844), available in Stülke lab
- pGP230 (amino acids 1-60, RNA-binding domain with thrombin cleavage site, in pWH844), available in Stülke lab
- pGP164 (both PRDs, in pWH844), in addition diverse expression vectors for phosphorylation site mutants and for RBD mutants (all in pWH844), available in Stülke lab
- pGP424 (PRDI, in pWH844), available in Stülke lab
- pGP425 (PRDII, in pWH844), available in Stülke lab
- pGP442 (PRDI, in pGP570, with thrombin cleavage site), available in Stülke lab
- pGP443 (PRDII, in pGP570, with thrombin cleavage site), available in Stülke lab
- pGP575 (amino acids 1-60, RNA-binding domain with Strep-tag, in pGP574), available in Stülke lab
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Original publications
Additional publications: PubMed