Difference between revisions of "Sandbox"
Raphael2215 (talk | contribs) |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' replication initiation protein <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''dnaA'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''dnaH, dnaJ, dnaK '' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || replication initiation protein |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || [[DNA replication]] |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/ | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/DnaA DnaA] |
|- | |- | ||
| − | | | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 50 kDa, 6.035 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| ''' | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1338 bp, 446 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| ''' | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[rpmH]]'', ''[[dnaN]]'' |
|- | |- | ||
| − | |style="background:# | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB11777]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | + | |colspan="2" | '''Genetic context''' <br/> [[Image:DnaA_dnaN_yaaA_recF_yaaB_gyrB_context.png]] | |
| − | |||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id= | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dnaA_410_1750_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:dnaA_expression.png|500px]] |
|- | |- | ||
|} | |} | ||
| Line 39: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| + | |||
| + | |||
| + | <br/><br/> | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| − | {{SubtiWiki category|[[ | + | {{SubtiWiki category|[[DNA replication]]}}, |
| − | {{SubtiWiki category|[[ | + | {{SubtiWiki category|[[essential genes]]}} |
| − | |||
| − | |||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| − | {{SubtiWiki regulon|[[ | + | {{SubtiWiki regulon|[[Spo0A regulon]]}} |
| − | + | =The [[DnaA regulon]]= | |
=The gene= | =The gene= | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | |
| + | * '''Locus tag:''' BSU00010 | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | ||
| − | + | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | |
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/dnaAN.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10065] |
=== Additional information=== | === Additional information=== | ||
| + | |||
=The protein= | =The protein= | ||
| Line 70: | Line 71: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' |
| − | * '''Protein family:''' | + | * '''Protein family:''' dnaA family (according to Swiss-Prot) |
* '''Paralogous protein(s):''' | * '''Paralogous protein(s):''' | ||
| Line 78: | Line 79: | ||
=== Extended information on the protein === | === Extended information on the protein === | ||
| − | * '''Kinetic information:''' | + | * '''Kinetic information:''' |
| − | * '''Domains:''' | + | * '''Domains:''' AAA+ domain |
| − | |||
| − | * '''Modification:''' | + | * '''Modification:''' |
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | ** | + | ** [[SirA]] displaces [[DnaA]] from the replication origin {{PubMed|19682252}} |
| + | ** [[YabA]] inhibits co-operative binding of DnaA to the ''oriC'' DNA {{PubMed|21895792}} | ||
| + | ** DnaA helix formation (and thus replication initiation) is inhibited by the interaction of [[Soj]] with the AAA+ domain of DnaA {{PubMed|22286949}} | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | ** | + | ** [[DnaA]] assembles into a right-handed helical oligomer built upon interactions between neighbouring AAA+ domains |
| − | ** | + | ** [[Soj]]-[[DnaA]] {{PubMed|22286949}} |
| − | ** [[ | + | ** [[DnaA]]-[[YabA]], [[DnaA]]-[[YabA]]-[[DnaN]] {{PubMed|12060778}} |
| − | ** [[ | + | ** [[DnaA]]-[[DnaD]] [http://www.ncbi.nlm.nih.gov/sites/entrez/11222620 PubMed] |
| − | ** [[ | + | ** [[SirA]]-[[DnaA]] {{PubMed|19682252,21239581}} |
| + | ** [[YqaH]]-[[DnaA]] {{PubMed|12060778}} | ||
| − | * '''[[Localization]]:''' | + | * '''[[Localization]]:''' throughout the cytoplasm [http://www.ncbi.nlm.nih.gov/sites/entrez/10844689 PubMed] |
| − | |||
| − | |||
| − | |||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' |
| − | |||
| − | |||
| − | * '''UniProt:''' [http://www.uniprot.org/uniprot/ | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/P05648 P05648] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu: | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU00010] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' |
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[dnaA]]-[[dnaN]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] |
| − | |||
| − | |||
| − | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id= | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dnaA_410_1750_1 dnaA] {{PubMed|22383849}} |
| − | * ''' | + | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] |
| − | * ''' | + | * '''Regulation:''' negatively controlled by [[DnaA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/2168872 PubMed] and [[Spo0A]] [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] |
| + | ** repressed under conditions that trigger sporulation ([[Spo0A]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] | ||
| − | * ''' | + | * '''Regulatory mechanism:''' |
| − | ** | + | ** [[Spo0A]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] |
| − | |||
| − | |||
| − | |||
| − | |||
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 143: | Line 132: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * '''Expression vector:''' | ||
| + | |||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| − | |||
| − | * '''GFP fusion:''' | + | * '''GFP fusion:''' |
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' |
| − | * ''' | + | * '''Antibody:''' |
| − | + | =Labs working on this gene/protein= | |
| + | [[Philippe Noirot]], Jouy-en-Josas, France [http://locus.jouy.inra.fr/cms/index.php?id=18 homepage] | ||
| − | + | [[Peter Graumann]], Freiburg University, Germany [http://www.biologie.uni-freiburg.de/data/bio2/graumann/index.htm homepage] | |
| − | [[ | + | [[Alan Grossman]], MIT, Cambridge, MA, USA |
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 176: | Line 155: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed> | + | <pubmed> 20157337 21035377 21639790</pubmed> |
| − | == | + | ==The [[DnaA regulon]]== |
| − | '''Additional publications:''' {{PubMed| | + | <pubmed> 16120674, </pubmed> |
| − | <pubmed> | + | ==Original publications== |
| − | + | '''Additional publications:''' {{PubMed|22396664,21911367}} | |
| − | + | <pubmed>18854156,19011033, 11222620,14651647,17140409 10844689 ,11222620 12060778 16461910 2987848 2168872 11207367, 19737352 19081080 17932079 19968790 19682252 20511500 21097613 21239581 21895792 22286949</pubmed> | |
| − | |||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:45, 18 April 2012
- Description: replication initiation protein
| Gene name | dnaA |
| Synonyms | dnaH, dnaJ, dnaK |
| Essential | yes PubMed |
| Product | replication initiation protein |
| Function | DNA replication |
| Interactions involving this protein in SubtInteract: DnaA | |
| MW, pI | 50 kDa, 6.035 |
| Gene length, protein length | 1338 bp, 446 aa |
| Immediate neighbours | rpmH, dnaN |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 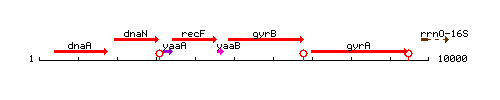
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The DnaA regulon
The gene
Basic information
- Locus tag: BSU00010
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: dnaA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: AAA+ domain
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: throughout the cytoplasm PubMed
Database entries
- Structure:
- UniProt: P05648
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Philippe Noirot, Jouy-en-Josas, France homepage
Peter Graumann, Freiburg University, Germany homepage
Alan Grossman, MIT, Cambridge, MA, USA
Your additional remarks
References
Reviews
The DnaA regulon
Original publications
Additional publications: PubMed