Difference between revisions of "RnpA"
(→Original Publications) |
(→Database entries) |
||
| Line 88: | Line 88: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1A6F 1AF6] [http://www.ncbi.nlm.nih.gov/sites/entrez/9563955 PubMed] | + | * '''Structure:''' |
| + | ** [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1A6F 1AF6] [http://www.ncbi.nlm.nih.gov/sites/entrez/9563955 PubMed] | ||
| + | ** [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=3OKB 3OKB] (complex of RnpA with the RNA RnpB and tRNA, from ''Thermotoga maritima'') {{PubMed|21076397}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P25814 P25814] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P25814 P25814] | ||
Revision as of 15:17, 15 December 2010
- Description: protein component of RNase P
| Gene name | rnpA |
| Synonyms | |
| Essential | yes PubMed |
| Product | protein component of RNase P (substrate specificity) |
| Function | cleavage of precursor sequences from the 5' ends of pre-tRNAs |
| MW, pI | 13 kDa, 10.804 |
| Gene length, protein length | 348 bp, 116 aa |
| Immediate neighbours | spoIIIJ, rpmH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 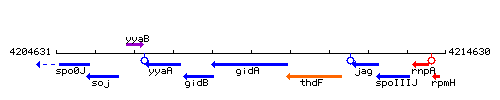
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
Rnases, translation, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU41050
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Endonucleolytic cleavage of RNA, removing 5'-extranucleotides from tRNA precursor (according to Swiss-Prot)
- Protein family: rnpA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure:
- UniProt: P25814
- KEGG entry: [2]
- E.C. number: 3.1.26.5
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Carol Fierke, University of Michigan, Ann Arbor, USA homepage
- Roland Hartmann, Marburg University, Germany homepage
Your additional remarks
References
Reviews
Original Publications
Additional publications: PubMed