Difference between revisions of "PrkC"
(→Database entries) |
(→References) |
||
| Line 128: | Line 128: | ||
=References= | =References= | ||
| + | ==Reviews== | ||
| + | <pubmed> 20972452 </pubmed> | ||
==Phosphorylation of PrkC== | ==Phosphorylation of PrkC== | ||
| − | <pubmed>12842463 | + | <pubmed>12842463 </pubmed> |
| + | '''Additional publications: ''' {{PubMed|20563625}} | ||
==Targets of PrkC-dependent phosphorylation== | ==Targets of PrkC-dependent phosphorylation== | ||
<pubmed>19246764, 20070526 ,20389117 </pubmed> | <pubmed>19246764, 20070526 ,20389117 </pubmed> | ||
==Phsiological role of PrkC== | ==Phsiological role of PrkC== | ||
<pubmed>12399479, 12406230, 19246764, 12842463 , 18984160 </pubmed> | <pubmed>12399479, 12406230, 19246764, 12842463 , 18984160 </pubmed> | ||
| − | ==Expression of PrkC== | + | ==Expression of PrkC: {{PubMed|16025310}}== |
| − | + | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:16, 26 October 2010
- Description: protein kinase C
| Gene name | prkC |
| Synonyms | yloP |
| Essential | no |
| Product | protein kinase |
| Function | germination in response to muropeptides |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 71 kDa, 4.833 |
| Gene length, protein length | 1944 bp, 648 aa |
| Immediate neighbours | prpC, cpgA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 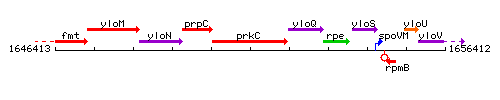
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU15770
Phenotypes of a mutant
- unable to germinate in response to muropeptides PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + a protein = ADP + a phosphoprotein (according to Swiss-Prot)
- Protein family: protein kinase domain (according to Swiss-Prot)
- Paralogous protein(s):
Proteins phosphorylated by PrkC
CpgA, EF-Tu, YezB PubMed, EF-G PubMed, YwjH, GlnA, Icd, AlsD, HPr PubMed
Extended information on the protein
- Kinetic information:
- Domains: PASTA domain at the C-terminus (binds muropeptides) PubMed
- Modification: phosphorylation on Thr-290 PubMed, autophosphorylation on multiple threonine residues PubMed
- Cofactor(s):
- Effectors of protein activity: activated by muropeptides PubMed
- Interactions:
Database entries
- Structure:
- UniProt: O34507
- KEGG entry: [2]
- E.C. number: 2.7.11.1
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP576 (spc), OMG302 (aphA3), available in Stülke lab
- Expression vector:
- for expression/ purification from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP832, available in Stülke lab
- for expression/ purification of the kinase domain from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP849, available in Stülke lab
- for expression, purification in E. coli with N-terminal His-tag, in pWH844: pGP1001, available in Stülke lab
- for expression, purification in E. coli with N-terminal Strep-tag, in pGP172: pGP825, available in Stülke lab
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Phosphorylation of PrkC
Additional publications: PubMed
Targets of PrkC-dependent phosphorylation
Phsiological role of PrkC