Difference between revisions of "CggR"
(→References) |
(→Reviews) |
||
| Line 129: | Line 129: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed> | + | <pubmed> 20408793 </pubmed> |
| + | |||
==Original Publications== | ==Original Publications== | ||
<pubmed>19193632,12850135,12622823,18186488,10799476,11489127,12123463,12634343, 18554327, 18052209 , 17293407 20361740 </pubmed> | <pubmed>19193632,12850135,12622823,18186488,10799476,11489127,12123463,12634343, 18554327, 18052209 , 17293407 20361740 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:08, 23 April 2010
- Description: repressor of the glycolytic gapA operon, DeoR family
| Gene name | cggR |
| Synonyms | yvbQ |
| Essential | no |
| Product | central glycolytic genes regulator |
| Function | transcriptional regulator |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 37,2 kDa,5.68 |
| Gene length, protein length | 1020 bp, 340 amino acids |
| Immediate neighbours | gapA, araE |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 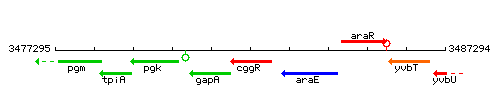
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU33950
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription repression of the glycolytic gapA operon
- Protein family: sorC transcriptional regulatory family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- DNA binding domain (H-T-H motif) (37–56)
- Modification:
- Cofactor(s):
- Effectors of protein activity: fructose 1.6-bisphosphate PubMed and dihydroxyacetone phosphate, glucose-6-phosphate and fructose-6-phosphate PubMed act as inducer and result in release of CggR from the DNA
- Interactions:
- Localization:
Database entries
- Structure: 2OKG ( effector binding domain), 3BXH (in complex with fructose-6-phosphate), complex with Fructose-6-Phosphate NCBI, effector binding domain NCBI
- UniProt: O32253
- KEGG entry: [3]
Additional information
Expression and regulation
- Database entries: DBTBS
- Additional information:
Biological materials
- Mutant:
- GP311 (in frame deletion), available in Stülke lab
- SM-NB7 (cggR-spc), available in Anne Galinier's and Boris Görke's labs
- GFP fusion:
- Antibody: available in Stülke lab
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Your additional remarks
References
Reviews
Original Publications