Difference between revisions of "TrpA"
(→Database entries) |
(→Database entries) |
||
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1WQ5 1WQ5] (from ''Escherichia coli'', | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1WQ5 1WQ5] (from ''Escherichia coli'', 35% identity, 53% similarity) {{PubMed|15667212}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P07601 P07601] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P07601 P07601] | ||
Revision as of 11:20, 19 February 2010
- Description: tryptophan synthase (alpha subunit)
| Gene name | trpA |
| Synonyms | |
| Essential | no |
| Product | tryptophan synthase (alpha subunit) |
| Function | biosynthesis of tryptophan |
| Metabolic function and regulation of this protein in SubtiPathways: Phe, Tyr, Trp | |
| MW, pI | 29 kDa, 4.817 |
| Gene length, protein length | 801 bp, 267 aa |
| Immediate neighbours | hisC, trpB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 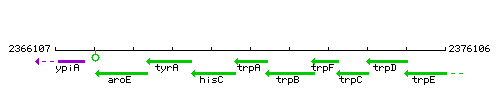
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU22630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-serine + 1-C-(indol-3-yl)glycerol 3-phosphate = L-tryptophan + glyceraldehyde 3-phosphate + H2O (according to Swiss-Prot)
- Protein family: UPF0403 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: P07601
- KEGG entry: [3]
- E.C. number: 4.2.1.20
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Gintaras Deikus, Paul Babitzke, David H Bechhofer
Recycling of a regulatory protein by degradation of the RNA to which it binds.
Proc Natl Acad Sci U S A: 2004, 101(9);2747-51
[PubMed:14976255]
[WorldCat.org]
[DOI]
(P p)
J Otridge, P Gollnick
MtrB from Bacillus subtilis binds specifically to trp leader RNA in a tryptophan-dependent manner.
Proc Natl Acad Sci U S A: 1993, 90(1);128-32
[PubMed:8419914]
[WorldCat.org]
[DOI]
(P p)
P Babitzke, P Gollnick, C Yanofsky
The mtrAB operon of Bacillus subtilis encodes GTP cyclohydrolase I (MtrA), an enzyme involved in folic acid biosynthesis, and MtrB, a regulator of tryptophan biosynthesis.
J Bacteriol: 1992, 174(7);2059-64
[PubMed:1551827]
[WorldCat.org]
[DOI]
(P p)
H Shimotsu, M I Kuroda, C Yanofsky, D J Henner
Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon.
J Bacteriol: 1986, 166(2);461-71
[PubMed:2422155]
[WorldCat.org]
[DOI]
(P p)
D J Henner, L Band, H Shimotsu
Nucleotide sequence of the Bacillus subtilis tryptophan operon.
Gene: 1985, 34(2-3);169-77
[PubMed:3924737]
[WorldCat.org]
[DOI]
(P p)
H Shimotsu, D J Henner
Characterization of the Bacillus subtilis tryptophan promoter region.
Proc Natl Acad Sci U S A: 1984, 81(20);6315-9
[PubMed:6436812]
[WorldCat.org]
[DOI]
(P p)