Difference between revisions of "BkdAA"
(→References) |
(→Database entries) |
||
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore/explore.do?pdbId=1UMB 1UMB] (from '' Thermus thermophilus'', 42% identity, 57% similarity) {{PubMed|15033367}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P37940 P37940] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P37940 P37940] | ||
Revision as of 11:28, 4 February 2010
- Description: 2-oxoisovalerate dehydrogenase (E1 alpha subunit)
| Gene name | bkdAA |
| Synonyms | bfmBAA, bfmB1a, bkd |
| Essential | no |
| Product | 2-oxoisovalerate dehydrogenase (E1 alpha subunit) |
| Function | utilization of branched-chain keto acids |
| Metabolic function and regulation of this protein in SubtiPathways: Ile, Leu, Val | |
| MW, pI | 36 kDa, 4.778 |
| Gene length, protein length | 990 bp, 330 aa |
| Immediate neighbours | bkdAB, lpdV |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 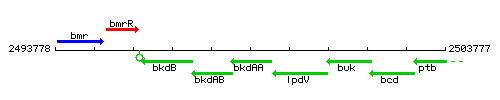
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU24050
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 3-methyl-2-oxobutanoate + [dihydrolipoyllysine-residue (2-methylpropanoyl)transferase] lipoyllysine = [dihydrolipoyllysine-residue (2-methylpropanoyl)transferase] S-(2-methylpropanoyl)dihydrolipoyllysine + CO2 (according to Swiss-Prot)
- Protein family: BCKDHA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: Membrane-proximal (Spotty) PubMed
Database entries
- UniProt: P37940
- KEGG entry: [3]
- E.C. number: 1.2.4.4
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
M Nickel, G Homuth, C Böhnisch, U Mäder, T Schweder
Cold induction of the Bacillus subtilis bkd operon is mediated by increased mRNA stability.
Mol Genet Genomics: 2004, 272(1);98-107
[PubMed:15241682]
[WorldCat.org]
[DOI]
(P p)
Ken-ichi Yoshida, Hirotake Yamaguchi, Masaki Kinehara, Yo-hei Ohki, Yoshiko Nakaura, Yasutaro Fujita
Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box.
Mol Microbiol: 2003, 49(1);157-65
[PubMed:12823818]
[WorldCat.org]
[DOI]
(P p)
Tanja Kaan, Georg Homuth, Ulrike Mäder, Julia Bandow, Thomas Schweder
Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response.
Microbiology (Reading): 2002, 148(Pt 11);3441-3455
[PubMed:12427936]
[WorldCat.org]
[DOI]
(P p)
M Debarbouille, R Gardan, M Arnaud, G Rapoport
Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis.
J Bacteriol: 1999, 181(7);2059-66
[PubMed:10094682]
[WorldCat.org]
[DOI]
(P p)
T Kaneda
Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance.
Microbiol Rev: 1991, 55(2);288-302
[PubMed:1886522]
[WorldCat.org]
[DOI]
(P p)