Difference between revisions of "Hmp"
| Line 95: | Line 95: | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| + | ** repressed unless NO is present under anaerobic conditions ([[NsrR]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/16885456 PubMed] | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[NsrR]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/16885456 PubMed] | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 16:56, 11 June 2009
- Description: flavohemoglobin, involved in resistance to nitric oxide (NO)
| Gene name | hmp |
| Synonyms | ykiA |
| Essential | no |
| Product | flavohemoglobin |
| Function | resistance to NO |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 44 kDa, 5.705 |
| Gene length, protein length | 1197 bp, 399 aa |
| Immediate neighbours | ykhA, ykzH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 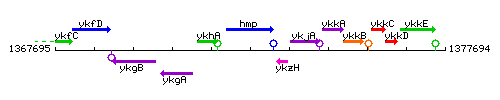
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU13040
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 NO + 2 O2 + NAD(P)H = 2 NO3- + NAD(P)+ (according to Swiss-Prot)
- Protein family: FAD-binding FR-type domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P49852
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Hochgräfe F, Wolf C, Fuchs S, Liebeke M, Lalk M, Engelmann S, Hecker M. (2008) Nitric oxide stress induces different responses but mediates comparable protein thiol protection in Bacillus subtilis and Staphylococcus aureus. J Bacteriol. Jul;190(14): 4997-5008. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed