Difference between revisions of "PhoR"
(→References) |
|||
| Line 37: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 74: | Line 70: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 94: | Line 87: | ||
* '''Domains:''' two transmembrane segments, C-terminal histidine phosphotransferase domain | * '''Domains:''' two transmembrane segments, C-terminal histidine phosphotransferase domain | ||
| − | * '''Modification:''' autophosphorylation on a His residue | + | * '''Modification:''' autophosphorylation on a His residue in response to the the availability of an intermediate of wall teichoic acid bioynthesis, autophosphorylation is prevented by binding of this intermediate to the intracellular [[PAS domain]] of [[PhoR]] {{PubMed 25315493}} |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 100: | Line 93: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''[[SubtInteract|Interactions]]:''' [[PhoP]]-[[PhoR]] | + | * '''[[SubtInteract|Interactions]]:''' |
| + | ** [[PhoP]]-[[PhoR]] | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 107: | Line 101: | ||
* '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU29100&redirect=T BSU29100] | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU29100&redirect=T BSU29100] | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=3CWF 3CWF] (extracellular | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=3CWF 3CWF] (extracellular domain) {{PubMed|20008068}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P23545 P23545] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P23545 P23545] | ||
| Line 159: | Line 153: | ||
=References= | =References= | ||
| − | <pubmed>20008068 10433720,15205429,9084179,17085571,9987123,10913081,10094672,16452408 20167622 ,15205429,14762014, 20382764 25315493 </pubmed> | + | <pubmed>20008068 10433720,15205429,9084179,17085571,9987123,10913081,10094672,16452408 20167622 ,15205429,14762014, 20382764 25315493</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:55, 27 October 2014
- Description: two-component sensor kinase, regulation of phosphate metabolism
| Gene name | phoR |
| Synonyms | |
| Essential | no |
| Product | two-component sensor kinase |
| Function | regulation of phosphate metabolism |
| Gene expression levels in SubtiExpress: phoR | |
| Interactions involving this protein in SubtInteract: PhoR | |
| MW, pI | 64 kDa, 5.957 |
| Gene length, protein length | 1737 bp, 579 aa |
| Immediate neighbours | polA, phoP |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 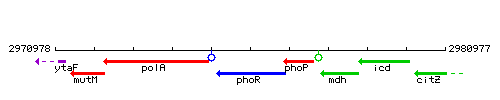
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed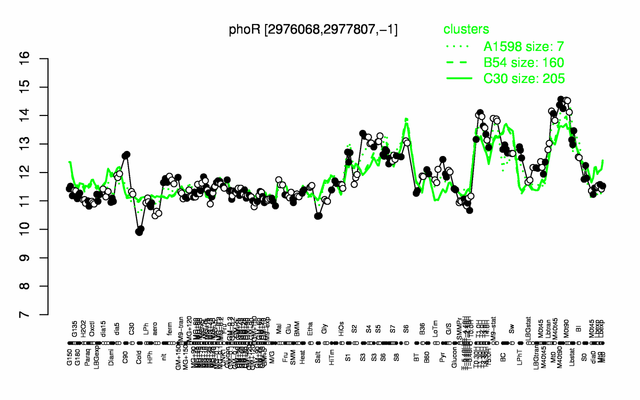
| |
Contents
Categories containing this gene/protein
phosphate metabolism, protein modification, transcription factors and their control, sporulation proteins, general stress proteins (controlled by SigB), membrane proteins, phosphoproteins
This gene is a member of the following regulons
CcpA regulon, PhoP regulon, SigB regulon, SigE regulon
The gene
Basic information
- Locus tag: BSU29100
Phenotypes of a mutant
Database entries
- BsubCyc: BSU29100
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of PhoP
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: two transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue in response to the the availability of an intermediate of wall teichoic acid bioynthesis, autophosphorylation is prevented by binding of this intermediate to the intracellular PAS domain of PhoR Template:PubMed 25315493
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU29100
- UniProt: P23545
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 453 PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Marion Hulett, University of Illinois at Chicago, USA Homepage
Your additional remarks
References